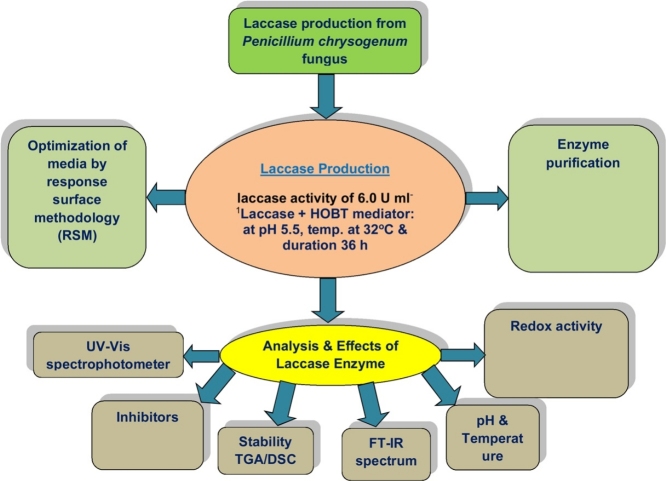
Graphical abstract
Keywords: Laccase, Penicillium chrysogenum, RSM, redox potential, UV-vis spec, DSC and FT-IR
Highlights
-
•
Isolation of Penicillium chrysogenum and production of Laccase there by.
-
•
Optimization of Operating conditions by central-composite-rotable-design / RSM.
-
•
Mol. wt of Laccase 67 k Da, UV spectra confirms type I Cu (II) & type III binuclear Cu (II) in enzyme.
-
•
FTIR confirms presence of amide I band, amide II band and amino acid in laccase.
-
•
Laccase gives hydrolytic de-gradation of chemical pollutants.
Abstract
This paper describes the isolation of potent extracellular-laccase producing white-rot fungus, identified by 18 s-rRNA as Penicillium-chrysogenum and its medium optimization by central-composite-rotatable-design using RSM. The optimum laccase-activity of 6.0 U ml-1 was obtained and maximum activity of 7.9 U ml-1 was achieved by statistical-optimization of the medium at 32 °C for 5 days. The molecular-weight of the laccase was found to be 67 kDa. UV-visible absorption-spectrum analysis shows peak at 600 nm and 325 nm corresponding to the type-I Cu(II) & type-III binuclear Cu(II) pair respectively confirming presence of laccase. The sharp endothermic peak at 150 °C and three-phases of protein denaturation was observed by DSC and TGA analysis for enzyme protein. The FT-IR analysis of laccase shows band at 1405cm-1, 1656 cm-1 &3400cm-1 corresponding to amide-I band, amide-II band and amino-acid group respectively. Results of the study show the enzyme is capable of carrying-out hydrolytic-cleavage of chemical-pollutants from the industrial waste-water for providing sustainable-greener environment
1. Introduction
Laccases are the multicopper blue oxidase type of enzymes which are called benzenediol: oxygen oxidoreductases, EC 1.10.3.2 as per systematic name. It has broader specificity towards substrates and can hydrolyze broad range of organic and inorganic compounds including phenolic groups, non-phenolic groups, hydroxyl and aromatic amine [1,2] with the reduction of molecular oxygen to water with the one electron oxidation mechanism (Thurston, 1994; Piontek et al., 2002). The laccase can be isolated from plants, insects and by microbes e.g. fungi and bacteria (Leonowicz et al., 2001). The laccase was first discovered in Japanese laquer tree Rhus vernicifera discovered by Yoshida in 1883 and first fungal laccase was reported by Bertrand and Laborde in 1896. High quantity of laccase enzyme could be obtained from fungal origin. The major laccase producing fungal strain belongs to the family of deuteromycetes, ascomycetes, and basidiomycetes, among these groups’ basidiomycetes are dominant laccase producer (Revankar and Lele, 2006; Kiiskinen et al., 2004). The white-rot fungi such as Trametes versicolor, Trametes hirsute, Trametes ochracea, Trametes villosa, Trametes gallica, Trametes trogii, Pycnoporus sanguineus, Cerena maxima, Coriolposis polyzona, Lentinus tigrinus, Pleurotus eryngii, etc., (Morozova et al., 2007; [3]; [4]; Birhanli et al., 2010).
Several literature reports that laccase enzyme is either monomeric or dimeric or multimeric glycoproteins in structural terms and may include variable carbohydrate content in the structure of the laccase (Wood, 1980). Most of the fungal laccases are extracellular monomeric globular proteins contains molecular weight around 50-130 kDa with an acidic isoelectric point (pI) around pH 4.0 ([5,6]; Johannes and Majcherczjk, 2000; [7]).
Laccase possesses broad catalytic properties; therefore it was exploited for wide biotechnological applications in various industrial areas. Laccase find application as health care field in medical diagnosis; and preparation of anticancer drug in pharmaceutical industry; synthesis of hormone derivatives, antiviral derivatives and antioxidants ([8]; Rocasalbas et al., 2013). The most interesting study by the application of laccase is the development of newer oxygen cathode in biofuel cells [9], biosensors, labeling in immunoassays and organic synthesis through biocatalysis [10], food industry [11], reduction of toxicity in cosmetics and dermatological preparations ([12]; [13]). The laccase applications are further extended to environmental biotechnology areas for biodegradation of xenobiotic compounds [14] like pulp delignification [15] and textile dye decolourisation and degradation of phenols, biphenyls, and applications in the agriculture field for clearing pesticides, herbicides, insecticides and explosives in soil by laccases with help of redox-mediator or without redox-mediator ([16]; [17]; Nyanhongo et al., 2002; Pickard et al., 1999).
Laccases are potential enzymes for treating various chemicals that cause environmental pollution in the processing industries. The merits of laccase suggest the researchers to produce in large scale for various applications. In our present study the new fungal strain was isolated from tree by tissue culture technique identified as Penicillium chrysogenum was utilized for the present study. The fungal strain was screened for laccase production using ABTS, guaiacol and tannic acid as a substrate, which resulted positive indication for laccase production. The laccase was characterized and the activity was studies in this present investigation. One activity unit is defined as the amount of enzyme that oxidizes 1 μmol of ABTS per min at 25 °C and the activities are expressed in U/L. Laccase activity was also determined spectrophotometrically at 465 nm with 4 mM guaiacol as a substrate in a reaction mixture containing 100 mM phosphate buffer, 0.3 ml enzyme extract incubated at 25 °C for 2 h.
2. MATERIALS AND METHODS
2.1. Chemicals
Guaiacol, ABTS, gallic acid, tannic acid, Potato Dextrose Agar (PDA) medium, Sabouraud Dextrose Agar (SDA) medium and agar powder. Unless otherwise stated all chemicals and microbial media used in this studies were procured from Sigma-Aldrich and Hi-Media, Mumbai, India and were certified reagent grade.
2.2. Isolation of fungal strain
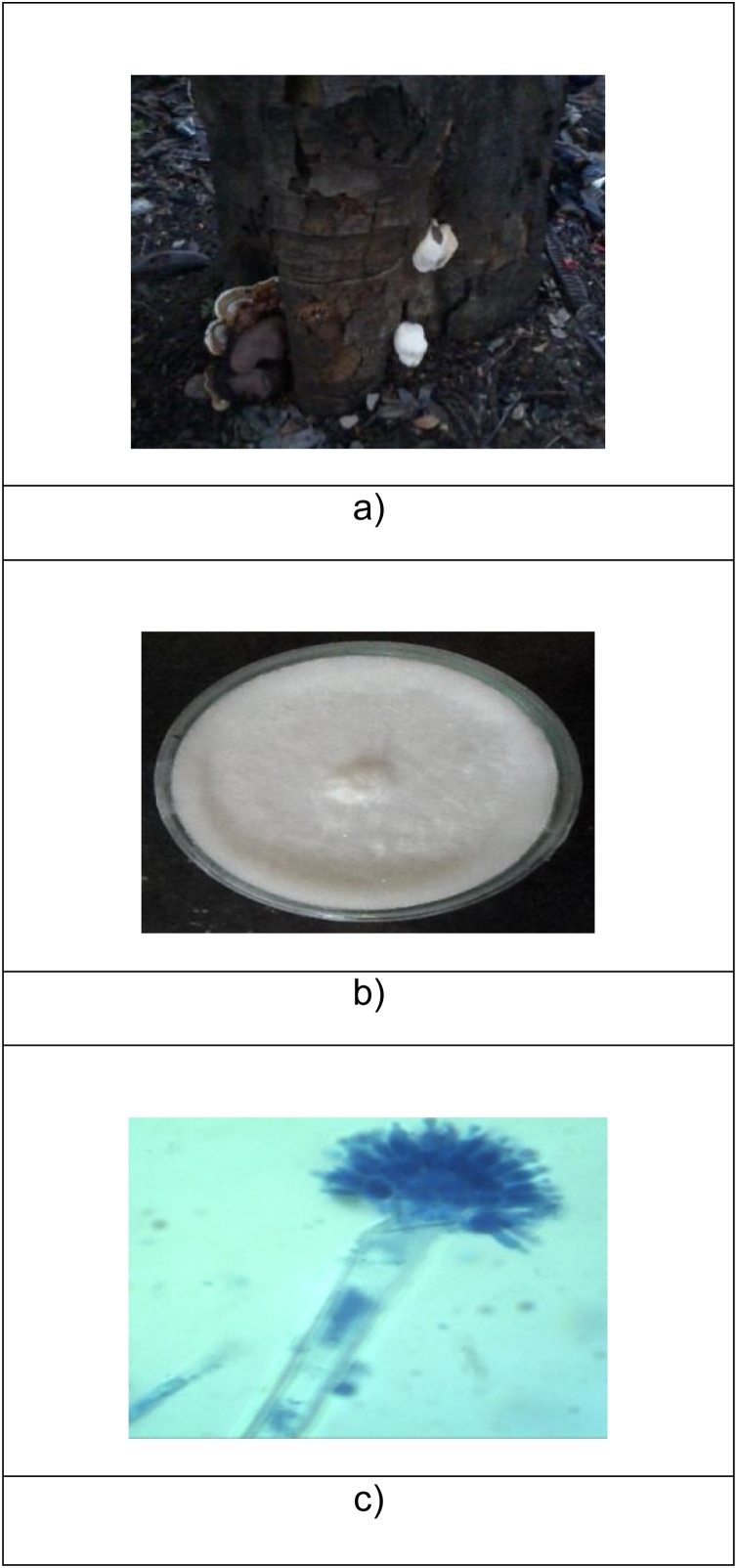
The fungi were isolated from tree by applying tissue culture technique according to the method described by Mishra et al. [18]. The fungi growing on tree was removed and washed thoroughly with running tap water and then surface was sterilized using 0.1% mercuric chloride for 30 s under aseptic condition. Further the fungus was washed with sterilized distilled water for three to four times, then sterilized with 75% alcohol under aseptic conditions for 30 s and then washed with distilled water after that inoculated into SDA medium. Further sub cultured as pure mycelial culture on PDA medium. Distinct and predominant fungal colonies were taken for the laccase production. White-rot fungus was isolated from bark of tree b). white-rot fungal growth, c) Microscopic view as shown in Fig. 1.
Fig. 1.
a). White-rot fungus was isolated from bark of tree b). white-rot fungal growth, c) Microscopic view.
2.3. Screening of fungi for laccase production
2.3.1. Plate assay method
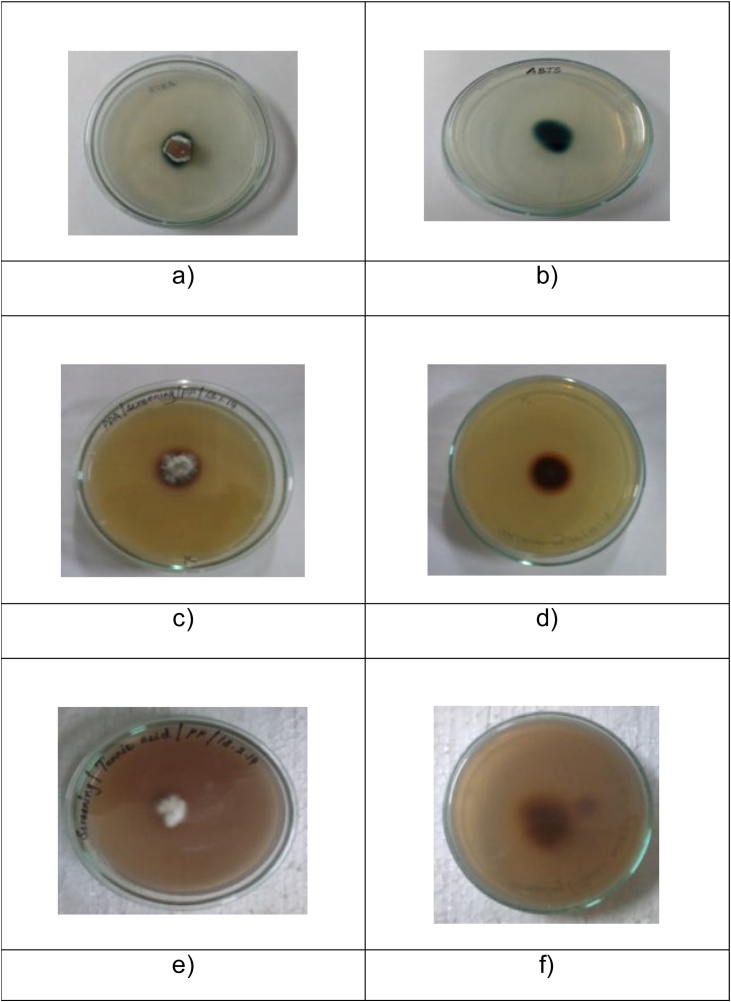
The isolated fungi were screened for laccase enzyme using ABTS (2-2’-Azino-bis-[3-ethyl benzthiazoline-6-sulfonic acid]), guaiacol, and tannic acid as a substrates. The fungus was inoculated on different PDA agar plates containing 3 mM of ABTS (Srinivasan et al., 1995; Prabu et al., 2006), 4 mM of guaiacol (Collins and Dobson, 1997) and 4 mM of tannic acid as individually and incubated at room temperature for 7 days. The culture plates were observed for color change in the media. Various substrates used for screening of Penicillium chrysogenum for laccase production, Fig. 2(a)&(b) front and back morphology of ABTS screening-green zone formed, Fig. 2(C) & (d) front and back morphology of guaiacol screening-reddish brown oxidation zone, Fig. 2(e) & (f) front and back morphology of tannic acid screening-dark brown oxidation zone
Fig. 2.
Various substrates used for screening of Penicillium chrysogenum for laccase production, a)&b) front and back morphology of ABTS screening-green zone formed, C)&d) front and back morphology of guaiacol screening-reddish brown oxidation zone, e)&f) front and back morphology of tannic acid screening-dark brown oxidation zone.
2.3.2. Bavendam test
Three-four fungal agar plugs were inoculated in to test tube containing potato dextrose broth supplemented with 10 mM guaiacol (ortho-methoxy phenol). The color change was observed in fungal culture broth for presence of laccase (Soponsathien, 1998).
2.4. Molecular Identification of fungi
The laccase positive fungi was identified by based on staining technique and 18 s rRNA sequencing method following the method described by Tamura et al. (2007). The 18 s rRNA sequencing analysis was carried out at Xcelris labs limited, Ahmedabad, India.
2.5. Production of laccase enzyme
The production of laccase enzyme was initially carried out in a liquid culture medium [19] with the modified medium components of (g/l): urea, 0.14; sucrose, 2.0; yeast extract, 0.34; MgSO4 ·7H2O, 0.07; CaCl2 ·2H2O, 0.004; NiSO4 ·7H2O, 0.003; KH2PO4, 0.1; and Na2HPO4, 0.3). Consecutive optimization studies were investigated by the classical one-factor-at-a-time method using various organic nitrogen sources (yeast extract, beef extract, meat extract, peptone, soybean meal and tryptone, 0.5–2.0%), inorganic nitrogen sources (NH4NO3, (NH4)2SO4, NH4Cl, KNO3, and NaNO3, 0.5–1.0%) and carbon sources (glucose, fructose, sucrose, maltose, lactose, and starch, 0.5–2.0%) for the production of urease.
2.6. Production media
The white-rot fungus was cut into small discs (5 mm size) after 7 days growth on PDA plates used for laccase production. About 10 PDA agar discs containing fungi mycelia were transferred to 250 ml erlenmeyer flasks containing 50 ml of liquid culture medium was initially used for laccase production with the media compositions of (g/l): glucose-20; peptone-5; ammonium tartarate-10; yeast extract-1.0; KCl-0.5; KH2PO4-1; MgSo4.7H2O-0.5; CuSo4.5H2O-0.25; and pH adjusted to 5.5 was used for laccase production (Zeng et al., 2012). Consecutive optimization studies were carried by the classical one-factor-at-a-time method using different carbon sources (manitol, lactose, maltose, glucose, sucrose and fructose) and different organic and inorganic nitrogen sources (sodium nitrate, peptone, beef extract, ammonium sulphate and ammonium chloride) for the production of fungal laccase enzyme (Niebisch et al., 2010). All the culture flasks were incubated at 32 °C for 7 days on a rotary shaker at 120 rpm. Samples were withdrawn from culture flasks at every 24 h intervals filtered and centrifuged at 10000 rpm for 10 min and the supernatant was assayed for enzyme activity.
2.7. Laccase activity measurement
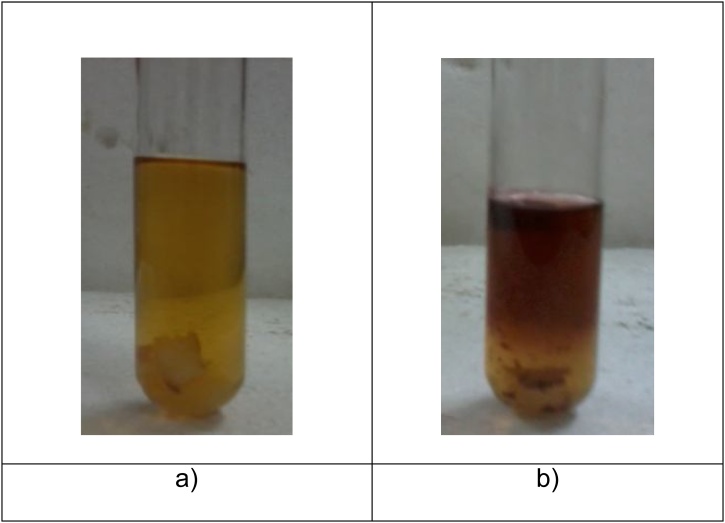
The laccase activity was measured by UV-vis spectrophotometer using guaiacol as a substrate. The reaction mixture consists of 3 ml of 100 mM of guaiacol dissolved in 10% acetone in sodium acetate buffer and 1 ml of culture filtrate (crude laccase). The mixture was incubated for 15 min and the absorbance was recorded at 470 nm. One unit of laccase activity was defined as the amount of enzyme catalyzing the substrate (guaiacol) for the production (Fig. 3) of 1 μl of colored product per min per ml (Collins and Dobson, 1997).
Fig. 3.
Deep brownish zone indicate the laccase activity by isolated fungal strain using guaicol as substrate. a) 0 h incubation, b) 48 h incubation [18].
2.8. Optimization of media composition using response surface methodology (RSM)
In order to find out the optimum media components for the fungal strain to obtain maximum laccase production, the media components were optimized by changing the nutrient composition. An elimination method was adapted to assess the effect of nutrient components that are essential for laccase production. As per the procedure, the media components obtained by the conventional one factor at-a-time approach were eliminated one at a time in each of the test media and the laccase activity after ach elimination was compared with the control wherein all components were present. Optimization of media composition was determined based on the concentration of protein content and maximum enzyme activity [20].
2.9. Factorial design and data analysis
On the basis of the results obtained by the classical method, five important critical variables were taken for the optimization by RSM. A five-level five factorial CCRD was performed in this study for the factors, pH (A), glucose (B), yeast extract (C), MgSO4 (D), CuSO4 (E) with an α-value ± 1.414. The relationship between the variation of the response, Yc (laccase activity, U ml-1) and the variation of factors A-E was represented by a second-order mathematical model using the following equation:
| Yc = β0 + β1X1 + β2X2 + β3X3 + β4X4 + β5X5 (intercept and main effects) |
| + β11X21 + β22X22 + β33X23 + β44X24 + β55X25 (interactions) |
| + β12X1 X2 + β13 X1X3 + β15X1 X5 |
| + β23X2 X3 + β24X2 X4 + β25X2 X5 + β34X3 X4 + β35X3 X5 + β45X4 X5 (quadratic effects) |
Where, Yc was the response calculated by the model and X1, X2, X3, X4 and X5 were the coded variables corresponding to factors A-E, respectively. Coding was required, since the factors were expressed in different units and β0 represented the regression coefficient at the centre. β1, β11, β12, β23, β34 and β45 were coefficients estimated by the model, which represented linear quadratic and interactive effects of X1, X2, X3, X4 and X5 factors on the response respectively. The treatment combination of CCRD was allocated in three blocks and first two blocks each had 20 runs and third block had 13 runs. The first two blocks each had sixteen factorial points and four centre points. Thus, in total, the experimental setup consisted of 53 trials and the value of the dependent response was the mean of triplicates.
Based on second-order equation, three dimensional response surface plots were drawn to illustrate the main and interactive effects of the independent variables on the dependent ones. They were drawn imposing constant values (i.e., the center points of the interval taken into consideration) to two of the independent variables of the CCRD. Statistical analysis of the model was performed using the “Design Expert” software package (version 7.0.0, Stat-Ease Inc., Minneapolis, USA) (Ramesh et al., 2013; [21,22]).
2.10. Purification of laccase
100 ml of crude laccase was partially purified by precipitation method using ammonium sulphate (80% saturation) and 70 % ice cold ethanol method. Enzyme active fraction were collected and centrifuged at 10000 rpm for 10 min and the pellet was dissolved in small amount of 0.1 M sodium acetate buffer solution and dialysed against the same buffer (0.01 M) overnight at 4 °C. The dialysed enzyme was further loaded on to sephadax G-100 on anion exchange column (1.5 x 30 cm), it was activated, washed and equilibrated with the same buffer. The enzyme elution was collected from the column at the flow rate of 0.5 ml/min with a linear gradient of NaCl (0.1-1.0 M) and the elution was monitored at 470 nm for laccase activity and 280 nm for measurement of protein by UV-vis spectrophotometer. The fraction containing laccase activity was collected and stored at 4 °C for further use (Vasdev et al., 2005; [23]; [18]; [24]). The protein content was estimated by Lowry’s method (Lowrys et al., 1951).
2.11. Molecular weight analysis using Sodium Dodecyl Sulfate-Poly Acryl amide Gel Electrophoresis (SDS-PAGE) technique
The molecular weight of the enzyme was determined by SDS-PAGE (Laemmli, 1970) under reducing and non reducing conditions with 10% polyacrylamide gel along with standard molecular weight marker (Jordan et al., 2004; [18]).
2.12. UV-Vis spectrophotometric analysis
The spectral study was carried out for purified laccase by UV-Vis Spectrophotometer (Hitachi U-2000 spectrophotometer) at wavelength from 250-700 nm. The 1 ml of enzyme dissolved in 2 ml of 0.1 M sodium acetate buffer, pH 5.0 [18].
2.13. Analysis of TGA, DSC and FT-IR
The purified lyophilized (freeze dried) laccase was subjected to the characterization by thermo gravimetric analysis (TGA), differential scanning colorimetry (DSC) and Fourier transform-infrared spectroscopy (FT-IR) analysis. The sample was analyzed by FT-IR spectrum at the range of 400–4000 cm-1 using a FT-IR Spectrum 2000 Perkin-Elmer spectrophotometer (Leonard and Lindley, 1998).
2.14. Analysis of redox potential
The purified laccase was subjected to the analysis of redox potential by cyclic voltametry. The cyclic voltameter was performed on a CH instruments electrochemical analyzer (model 680 AMP Booster) with a platinum disc-working electrode, an AgCl/ Ag reference electrode and a platinum wire counter electrode. Laccase was loaded in to the cell in anaerobic condition with high concentration of K3Mo (CN)8 in 0.1 M sodium acetate buffer at pH 5.0. The redox potential of the laccase was recorded using platinum electrode before and after addition of enzyme. In order to find out the catalytic activity and electric flow variation of the enzyme was recorded after addition of increasing concentration of substrates such as ABTS and syringaldazine. The voltammogram was recorded at the scanning rate of 0.1 V s-1 [25,26].
2.15. Effect of pH and temperature
The study was conducted for the purified laccase enzyme to find out the optimum pH conditions and temperature. The pH conditions of the enzyme was determined by keeping the enzyme at 4 °C for 1 h in different pH of buffer solution (0.1 M conc.) and the residual activity was calculated using standard assay conditions. The different buffer solution such as citrate buffer (pH at 2.0-3.5), acetate buffer (pH at 4.0-5.5), phosphate buffer (pH at 6.0-7.5), Tris-Hcl buffer (pH at 7.5-9.0) and carbonate-bicarbonate buffer (pH at 9.5-10.0). The temperature stability of the enzyme was determined at various temperature conditions ranges from 20-80 °C and the activity was measured using assay condition (0.1 M sodium acetate buffer, pH at 5.0) [25].
2.16. Effect of inhibitors on enzyme activity
The laccase has been tested against various potential inhibitors such as ethylene-diamine-tetraacetic acid (EDTA), thioglycolic acid, sodium azide (NaN3), ethanol and diethyldithiocarbamic acid (DDC) and laccase activity was measured under standard assay conditions. The reaction mixture consist of 2.4 ml of 0.1 M sodium acetate buffer containing guaiacol (100 mM pH 5.5) and 0.3 ml of enzyme (partially purified) and 0.3 ml of inhibitors were added at different concentrations and incubated at 32 °C. The reaction mixture was measured using UV-vis spectrophotometer at 470 nm and change in absorbance was recorded. Similar experiment was carried out for control in the absence of inhibitors ([25]; Mishra and Kumar, 2009).
2.17. Effect of metal ions on enzyme activity
To determine the effect of metal ions over the laccase activity, the reaction mixture consist of 0.3 ml of enzyme, 2.4 ml of 0.1 M sodium acetate buffer (pH 5.0) containing guaiacol (100 mM), 0.3 ml of metal ion solution at 32 °C. The metals such as Fe, Zn, Mg, Cr, Co and Hg were tested at the concentration ranges from 0.1-15 mM and incubated for 1 h. The residual enzyme activity was measured in reaction mixture after incubation. Similar experiment was performed for control with heat denatured enzyme solution. The IC50 (concentration required to inhibit 50% of laccase activity) of metal ions were determined.
Enzyme inhibition was calculated as follows:
Where, a0- activity without metal ions and ai- activity after incubation with metal ions [25,27].
3. Results and discussion
3.1. Isolation, screening and molecular identification of fungi
The laccase producing fungi were isolated from bark tree using tissue culture technique. It was inoculated on PDA medium supplemented with guaiacol (conc. 4 mM) as a substrate. The reddish brown oxidation zone was developed around the colonies which were taken for the laccase production. The laccase positive fungi were further sub cultured on PDA medium and evaluated for laccase production. Based on the initial higher laccase activity, one predominant white-rot fungus was selected for further study, among the laccase positive fungi. The white rot fungus was further screened for laccase production using ABTS (3 mM) and tannic acid (4 mM) as a substrate [18]. In plate assay method the green color was developed for medium containing ABTS as a substrate and dark brown color was developed for plate containing tannic acid as a substrate. The screening results indicating the color formation in the medium for the respective substrate confirm that isolated fungus can able to produce laccase enzyme.
The isolated fungus was further subjected to the morphological identification and staining technique. Based on the morphological identification and staining technique it is inferred that the isolated fungus belongs to the class of basidiomycetes white rot fungus. Further the fungus was identified as Penicillium chrysogenum by molecular identification of 18 s rRNA sequencing method [18].
3.2. Screening: Biochemical characterization (screening for laccase detection on culture plate)
The screening of laccase by culture plate method from Biochemical characterization / enzymatic activity was carried out and presented in the Fig. 3. It can be seen from the figure that a deep green zone around and above the isolated colony is formed. It is the confirmatory and positive test for the presence of laccase activity. The screened colony was further taken for the laccase production [18].
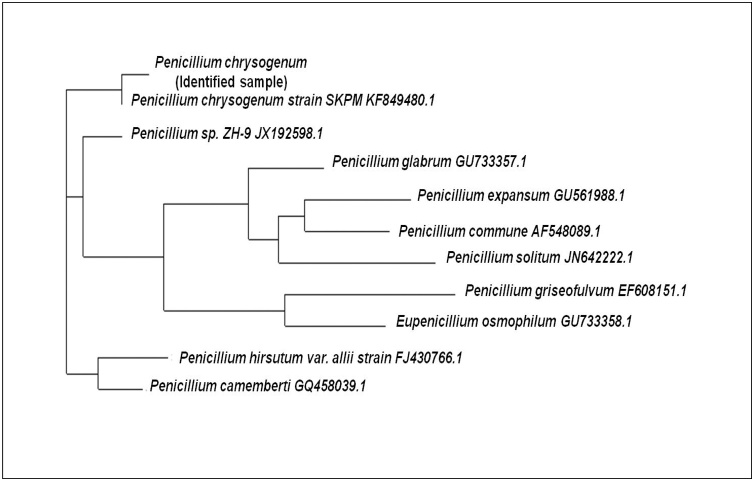
The molecular analysis of 18 s rRNA gene sequence and phylogenetic tree was constructed by neighbor-joining program using bootstrap consensus test with 100 replicates that confirmed that the fungus belongs to the category of Penicillium chrysogenum (Fig. 5). The Penicillium chrysogenum gene sequence was homologous to Penicillium chrysogenum strain SKPM KF849480.1 and Eupenicillium osmophilum GU733358.1 18 s rRNA sequence obtained from BLAST search with 99% similarity.
Fig. 5.
Phylogenetic tree analysis of 18 s rRNA gene sequence of Penicillium chrysogenum.
3.3. Laccase production
The laccase producing fungi that were screened and selected for laccase production, was grown on PDA slants for 5 days at 32 °C preserved and stored at 4 °C. From this; one predominant laccase producing white-rot fungus was selected among the various fungi and incubated at 32 °C for 5 days. The supernatant of the culture containing the enzyme showed an initial laccase activity of 3.2 U/ml indicating the extracellular nature of the laccase [18].
3.4. Molecular weight analysis of laccase enzyme
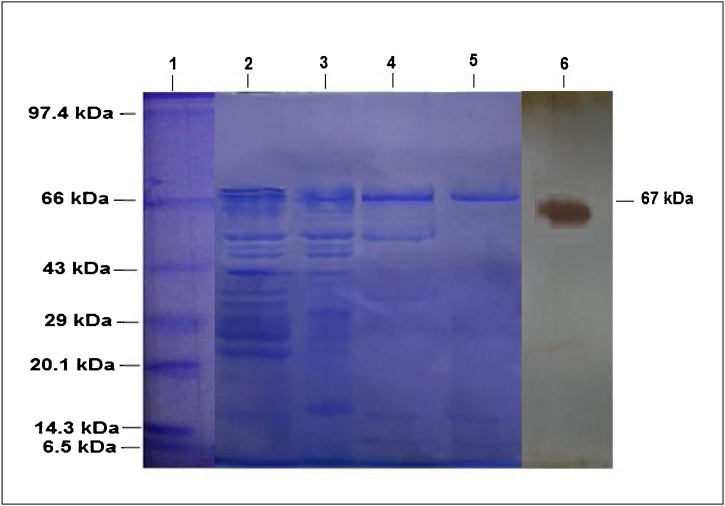
The molecular weight of purified laccase was found to be 68 k Da as visualized by commassie brilliant blue staining and (Fig. 6) this result were similar to the other laccase enzyme molecular weight range of 60-70 k Da as reported earlier [25,28,29]. However the molecular weight of the laccases found to be ranges between 50-130 kDa [[30], [31], [32], [33]]. A plausible mechanism of laccase interaction with Guaiacol during enzyme activity measurement by UV-vis spectrophotometer at 470 nm is shown in Fig. 4a, while that with ABTS is shown in Fig. 4b
Fig. 6.
SDS-PAGE Analysis for enzyme molecular weight determination. 1) Marker, 2). Crude, 3). Ammonium sulfate precipitation, 4). Gel Filtration, 5). DEAE-FF and 6) zymogram with guaiacol as a substrate.
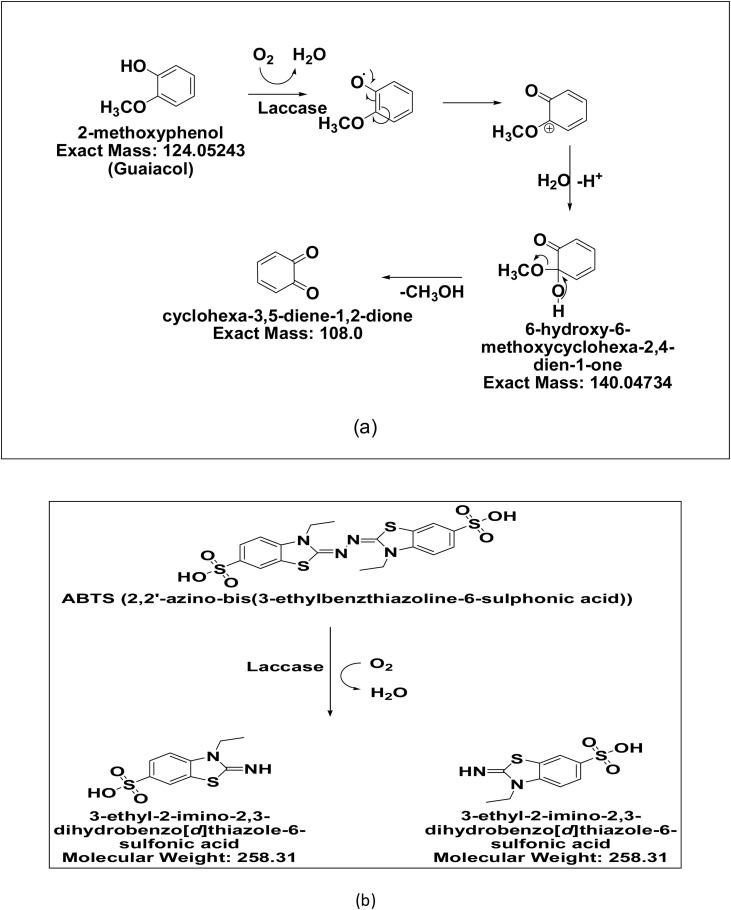
Fig. 4.
Plausible mechanism of laccase enzyme (a) interaction with Guaiacol during enzyme activity measurement by UV-vis spectrophotometer at 470 nm (b) reaction with ABTS.
3.5. Optimization using one-factor-at-a-time method
The production of laccase enzyme from Penicillium chrysogenum fungus was substantially enhanced by consecutive optimization of the media components. An optimal laccase activity was found to be 6 U/ml at 5 days by conventional one-factor-at-a-time approach in a medium containing (% w/v): glucose-17; peptone-4.5; ammonium tartarate-8.5; yeast extract-1.3 g; KCl-0.07; KH2PO4-0.8; MgSO4- 0.6; CuSO4-2 mM; and pH adjusted to 5.5. (Fig. 7). The laccase activity thus obtained was 1.875 times greater than the initial activity achieved from the liquid culture medium and the activity was further enhanced by statistical optimization of the medium components using central composite rotatable design (CCRD).
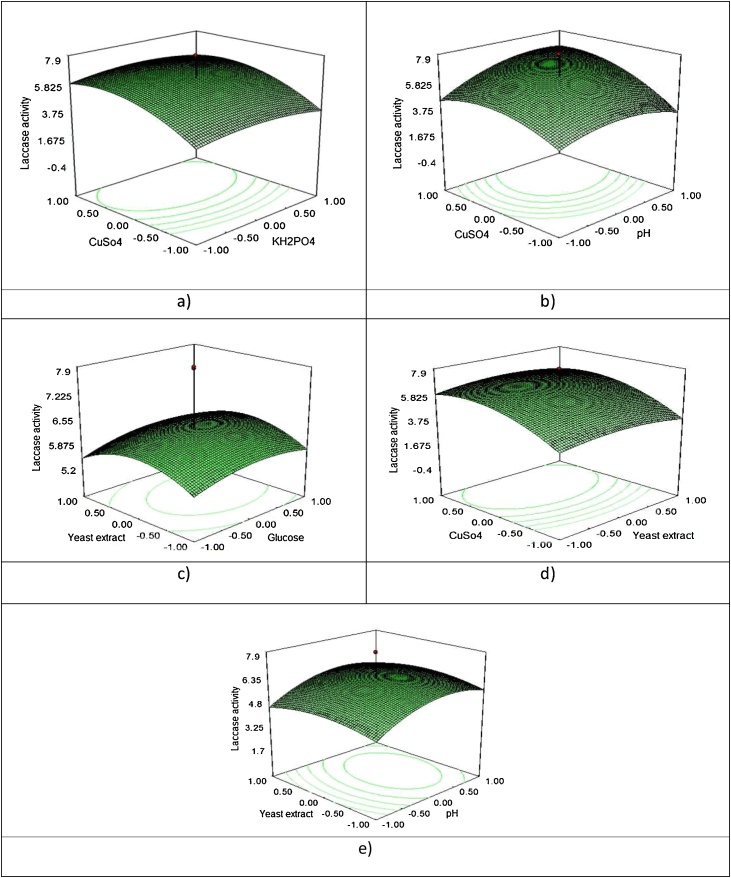
Fig. 7.
Response surface plot showing the interactive effect of (a) meat extract and urea (b) sucrose and urea and (c) Na2HPO4 and meat extract concentration on urease production by A. creatinolyticus.
3.6. Central composite rotatable design (CCRD) model fitting and ANOVA
CCRD was used to analyze the suitable concentrations of the variables (media components) for laccase production by Penicillium chrysogenum and the experimental domain depicting the levels for each selected variables (media components) are presented in Table 1. The levels of the variables were selected for production of laccase based on the obtained results from the conventional one-factor-at-a-time approach. The experiments were performed to obtain a quadratic model with five independent variables are presented in Table 1 and the data on the laccase production by Penicillium chrysogenum are presented in Table 2 and the predicted values obtained from the model fitting technique using the software Design-Expert version 7.0.0 are presented in Table 3. The correlation with the observed values and the quadratic polynomial model was seen to be highly significant to represent the actual relationship between the response (laccase activity) and the significant variables. The differences in laccase activities obtained from 1.03 to 7.9 U ml-1 indicated that the interactions among the nutritional factors played a more significant role than the effect of individual factors alone. The obtained results were further analyzed by standard analysis of variance (ANOVA). The ANOVA result for the quadratic regression model are presented in Table 3, it showed that the model was highly significant with an F value of 141.9845, which is evident from Fisher’s “F” test along with a very low probability value (p model > F = 0.0001). At the same time, a relatively lower coefficient of variation (CV = 7.28%) was indicated a better precision and reliability of the experiments carried out. The determination coefficient (R2) of the model was 0.9895 is explaining 98.95% of the variability in the response could be accounted for the model and only 1.05% of the total variation was not explained by the model ensuring a satisfactory adjustment of the quadratic model to the experimental data. Analysis of the design showed a high degree of fitting between predicted and experimental data, which indicated that the model suitably represented the real relationship among the selected factors. The insignificant lack of fit test also indicated that the model suitably represented the experimental data and the final predictive equation was as follows:
| Laccase activity (U/ml) = +6.30142 + 0.57695 (A) + 0.13956 (B) + 8.71076E-003 (C) + 0.019019 (D) + 1.04705 (E) +0.042906 (A)(B) + 0.028531(A)(C) + 0.036031 (A)(D) + 0.56603 (A)(E) + 0.038969 (B)(C) +0.03396 (B)(D) + 0.031469 (B)(E) + 0.033344 (C)(D) + 0.042094 (C)(E) + 0.029594 (D)(E) - 0.86632 (A)2 - 0.24229 (B)2 - 0.48271 (C)2 - 0.47476 (D)2 - 1.02895 (E)2 |
Table 1.
Experimental range of variables for the central composite rotatable design (CCRD)
| Variable | Symbol coded | Range of variables (g/l) |
||
|---|---|---|---|---|
| Low (-1) |
Mid (0) |
High (+1) |
||
| pH | A | 2.75 | 5.5 | 11 |
| Glucose | B | 8.5 | 17 | 34 |
| Yeast extract | C | 0.65 | 1.3 | 2.6 |
| KH2PO4 | D | 0.4 | 0.8 | 1.6 |
| CuSO4 | E | 1 mM | 2 mM | 4 mM |
Table 2.
Central composite rotatable design (CCRD)
| Run | Medium components |
Laccase activity (U/ml) |
||||||
|---|---|---|---|---|---|---|---|---|
| pH | Glucose | Yeast extract | KH2PO4 | CuSo4 | Experimental | Predicted | ||
| 1 | 2.75 | 34 | 0.65 | 1.6 | 0.2496 | 1.16 | ||
| 2 | 11 | 34 | 0.65 | 0.4 | 0.2496 | 1.25 | ||
| 3 | 5.5 | 17 | 1.3 | 0.8 | 0.4993 | 5.5 | ||
| 4 | 5.5 | 17 | 1.3 | 0.8 | 0.4993 | 5.45 | ||
| 5 | 2.75 | 34 | 0.65 | 0.4 | 0.9986 | 2.14 | ||
| 6 | 2.75 | 34 | 2.6 | 1.6 | 0.9986 | 2.347 | ||
| 7 | 11 | 34 | 0.65 | 1.6 | 0.9986 | 4.55 | ||
| 8 | 11 | 8.5 | 2.6 | 0.4 | 0.2496 | 1.15 | ||
| 9 | 11 | 8.5 | 2.6 | 1.6 | 0.9986 | 4.52 | ||
| 10 | 2.75 | 8.5 | 2.6 | 1.6 | 0.2496 | 1.17 | ||
| 11 | 2.75 | 8.5 | 0.65 | 1.6 | 0.9986 | 2.15 | ||
| 12 | 2.75 | 8.5 | 2.6 | 0.4 | 0.9986 | 2.19 | ||
| 13 | 5.5 | 17 | 1.3 | 0.8 | 0.4993 | 5.49 | ||
| 14 | 2.75 | 8.5 | 0.65 | 0.4 | 0.2496 | 1.53 | ||
| 15 | 2.75 | 17 | 2.6 | 0.4 | 0.2496 | 1.16 | ||
| 16 | 11 | 8.5 | 0.5 | 1.6 | 0.2496 | 1.24 | ||
| 17 | 11 | 8.5 | 2.6 | 1.6 | 0.2496 | 1.4 | ||
| 18 | 5.5 | 17 | 1.3 | 0.8 | 0.4993 | 5.46 | ||
| 19 | 11 | 8.5 | 0.65 | 0.4 | 0.9986 | 4.4 | ||
| 20 | 11 | 34 | 2.6 | 0.4 | 0.9986 | 4.55 | ||
| 21 | 11 | 34 | 0.65 | 1.6 | 0.2496 | 1.35 | ||
| 22 | 2.75 | 8.5 | 0.65 | 1.6 | 0.2496 | 1.35 | ||
| 23 | 2.75 | 8.5 | 2.6 | 0.4 | 0.2496 | 1.3 | ||
| 24 | 5.5 | 17 | 1.3 | 0.8 | 0.4993 | 5.51 | ||
| 25 | 5.5 | 17 | 1.3 | 0.8 | 0.4993 | 5.55 | ||
| 26 | 11 | 34 | 2.6 | 0.4 | 0.2496 | 1.32 | ||
| 27 | 2.75 | 34 | 0.65 | 0.4 | 0.2496 | 1.32 | ||
| 28 | 11 | 34 | 0.65 | 0.4 | 0.9986 | 4.42 | 2 | |
| 29 | 11 | 8.5 | 2.6 | 1.6 | 0.2496 | 1.29 | ||
| 30 | 11 | 8.5 | 0.65 | 1.6 | 0.9986 | 4.42 | ||
| 31 | 2.75 | 34 | 0.65 | 1.6 | 0.9986 | 2.25 | ||
| 32 | 5.5 | 17 | 1.3 | 0.8 | 0.4993 | 5.55 | 3 | |
| 33 | 11 | 34 | 2.6 | 1.6 | 0.9986 | 4.92 | ||
| 34 | 5.5 | 17 | 1.3 | 0.8 | 0.4993 | 5.55 | ||
| 35 | 2.75 | 34 | 2.6 | 1.6 | 0.2496 | 1.21 | ||
| 36 | 11 | 8.5 | 0.65 | 0.4 | 0.2496 | 1.38 | ||
| 37 | 2.75 | 8.5 | 2.6 | 1.6 | 0.9986 | 2.24 | ||
| 38 | 2.75 | 34 | 2.6 | 0.4 | 0.9986 | 2.25 | ||
| 39 | 11 | 8.5 | 2.6 | 0.4 | 0.9986 | 4.41 | ||
| 40 | 2.75 | 8.5 | 0.65 | 0.4 | 0.9986 | 2.33 | ||
| 41 | 5.5 | 17 | 1.3 | 0.8 | 0.4993 | 7.85 | ||
| 42 | 5.5 | 17 | 0.325 | 0.8 | 0.4993 | 5.29 | 5 | |
| 43 | 5.5 | 17 | 1.3 | 0.8 | 0.1053 | 1.03 | ||
| 44 | 5.5 | 0.575 | 1.3 | 0.8 | 0.4993 | 5.53 | ||
| 45 | 1.375 | 17 | 1.3 | 0.8 | 0.4993 | 1.79 | ||
| 46 | 5.5 | 17 | 1.3 | 0.8 | 2.3666 | 4.73 | ||
| 47 | 9.48 | 17 | 1.3 | 0.8 | 0.4993 | 4.53 | ||
| 48 | 5.5 | 17 | 1.3 | 0.8 | 0.4993 | 7.9 | 1 | |
| 49 | 5.5 | 17 | 1.3 | 9.48 | 0.4993 | 5.45 | ||
| 50 | 5.5 | 17 | 1.3 | 0.592 | 0.4993 | 5.3 | ||
| 51 | 5.5 | 17 | 5.2 | 0.8 | 0.4993 | 5.37 | ||
| 52 | 5.5 | 17 | 1.3 | 0.8 | 0.4993 | 7.85 | ||
| 53 | 5.5 | 68 | 1.3 | 0.8 | 0.4993 | 7.85 | ||
Results represented as mean ± SD of two independent experiments with three replicates each.
Table 3.
ANOVA for Response Surface Quadratic Model
| Source | Sum of Squares |
Degrees of freedom | Mean Square |
F-Value | p-value Prob > F |
|---|---|---|---|---|---|
| Block | 54.40473 | 2 | 27.20236 | ||
| Model | 189.2575 | 20 | 9.462877 | 141.9845 | < 0.0001 |
| A- pH | 14.41795 | 1 | 14.41795 | 216.3323 | < 0.0001 |
| B- Glucose | 0.84364 | 1 | 0.84364 | 12.65828 | <0.0001 |
| C- Yeast extract | 0.003287 | 1 | 0.003287 | 0.049312 | <0.0001 |
| D- KH2PO4 | 0.015668 | 1 | 0.015668 | 0.235082 | 0.6313 |
| E- CuSo4 | 47.48527 | 1 | 47.48527 | 712.4867 | < 0.0001 |
| AB | 0.05891 | 1 | 0.05891 | 0.883912 | 0.3546 |
| AC | 0.026049 | 1 | 0.026049 | 0.390849 | <0.0001 |
| AD | 0.041544 | 1 | 0.041544 | 0.623342 | 0.4360 |
| AE | 10.25252 | 1 | 10.25252 | 153.8327 | < 0.0001 |
| BC | 0.048594 | 1 | 0.048594 | 0.729123 | <0.0001 |
| BD | 0.036924 | 1 | 0.036924 | 0.554022 | 0.4625 |
| BE | 0.031689 | 1 | 0.031689 | 0.475474 | 0.4958 |
| CD | 0.035578 | 1 | 0.035578 | 0.533822 | 0.4707 |
| CE | 0.0567 | 1 | 0.0567 | 0.850752 | 0.3637 |
| DE | 0.028025 | 1 | 0.028025 | 0.420502 | 0.5216 |
| A^2 | 44.73592 | 1 | 44.73592 | 671.2345 | < 0.0001 |
| B^2 | 3.499361 | 1 | 3.499361 | 52.50572 | < 0.0001 |
| C^2 | 13.88919 | 1 | 13.88919 | 208.3987 | < 0.0001 |
| D^2 | 13.43518 | 1 | 13.43518 | 201.5865 | < 0.0001 |
| E^2 | 63.10923 | 1 | 63.10923 | 946.9145 | < 0.0001 |
| Residual | 1.999417 | 30 | 0.066647 | ||
| Lack of Fit | 1.99485 | 22 | 0.090675 | 158.8467 | 0.0511 |
| Pure Error | 0.004567 | 8 | 0.000571 | ||
| Cor Total | 245.6617 | 52 |
R2 = 0.9826; CV = 7.28%.
*Significant at prob. > F < 0.05.
It was clear that the four linear coefficients and eight quadratic coefficients were highly significant (p < 0.05) from the model and among the five variables, pH (A), Glucose (B), Yeast extract (C) and CuSo4 (D) were the most significant for laccase production, whereas KH2PO4 had a less significant effect on production of laccase. The high F value of 712.4867 is in addition to a least probability value (<0.0001) for copper sulphate, which is indicated that copper sulphate played a very prominent role in laccase production and enhanced laccase activity when compared to other variables. A significant quadratic regression model was expressed as an insignificant lack of fit and a small total variation (1.05%) and it was not explained by the model suggested that the model precisely represented the data in the experimental region.
The three-dimensional response surface graphs (Fig. 7a–e) were plotted to show the interaction among the medium components and the optimum concentration of each component required for better laccase production. The cumulative effect of meat KH2PO4 and CuSO4 concentrations on laccase production in a medium containing 17 g/l glucose and 1.3 g/l yeast extract at pH 5.5 is shown in the response surface plot of Fig. 7a. The laccase activity was 7.5 Uml-1, when the production medium contained 0.8 g/l KH2PO4 and 0.4993 g/l CuSO4, whereas decreasing the concentrations of KH2PO4 and CuSO4 drastically reduced the laccase production levels, confirming that KH2PO4 and CuSO4 had significant influence on laccase production. This activity was comparatively higher than the extracellular laccase activity of T. harzianum (4.36 U ml-1) at 4 days in a fermentation medium containing laccase KH2PO4and CuSO4 [25].
The interactive effect of pH and CuSO4 on the production of laccase is shown in Fig. 7b. Maximum laccase activity was obtained with 0.9986 g/l of CuSO4 and 5.5 pH concentrations. The cumulative effect of yeast extract and glucose (Fig. 7c) showed that the laccase activity was optimum, when the media contained moderate levels of yeast extract (1.3 g/l) with glucose (17 g/l). Further increasing concentrations of yeast extract led to decrease in the production of laccase. The cumulative effect of CuSO4 and yeast extract (Fig. 7d) showed that the laccase activity was optimum, when the media contained moderate levels of CuSo4 (0.4993 g/l) with yeast extract (1.3 g/l). The interactive effect of yeast extract and pH on the production of laccase is shown in Fig. 7e. Maximum laccase activity was obtained with 1.3 g/l of yeast extract and 5.5 pH concentrations. The maximal laccase activity (7.9 U ml-1) obtained under the optimized conditions (Table 2) was not depicted in the response surface plots. This could be due to the fact that response surface plots were drawn by imposing constant values (i.e., the central points of the interval taken into consideration) to two of the independent variables of the factorial design. The results of the RSM clearly showed that the influential parameters for the laccase production were 5.5 pH, 17 g/l glucose, 1.3 yeast extract, 0.8 g/l KH2PO4 and 0.4993 g/l CuSO4, with an activity of 7.9 U/ml at 5 days and 32 °C, which was about 2.46 times greater than the initial activity (3.2 U/ml) from the liquid culture medium.
3.7. Partial purification of laccase enzyme [18]
The extracellular laccase was harvested from the culture flasks by centrifugation at 10000 rpm for 10 min. The culture supernatant was partially purified to homogeneity by ammonium sulphate precipitation followed by dialysis and chromatographic separation was carried using DEAE cellulose column, in which laccase fraction was eluted from 0.1 M to 1.0 M NaCl gradient solution. The ammonium sulphate precipitation (60-80% saturation) method showed the maximum laccase activity was obtained at 70% saturation and the specific activity was calculated after this step was 1.26 U/mg with 1.57 fold purification. The main purification was done using DEAE cellulose column chromatography and after this step the calculated specific activity was found to be 25.30 and 3.42 fold purification (Table 4). The complete purification was obtained from culture supernatant by the DEAE cellulose column chromatography.
Table 4.
Purification of fungal laccase from Penicillium chrysogenum
| Purification steps | Activity (U/ml) | Protein (mg/ml) | Sp. activity (U/mg) | Yield (%) | Purification fold |
|---|---|---|---|---|---|
| Culture filtrate | 790 | 315 | 2.50 | 100 | 1 |
| Amm. Sul. precipitation | 724 | 78.46 | 9.22 | 91.64 | 3.69 |
| Dialysis | 643 | 26 | 24.73 | 81.39 | 9.89 |
| DEAE cellulose | 415 | 5.6 | 74.10 | 52.53 | 29.64 |
3.8. UV-vis spectrophotometric measurement of laccase enzyme
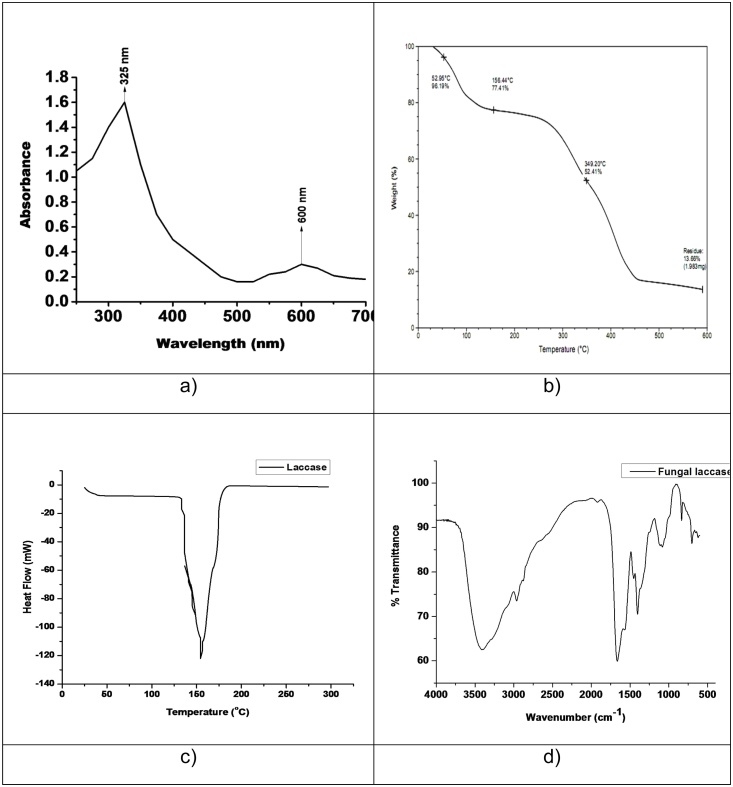
To determine the state of its catalytic center, the laccase was characterized using spectrophotometer. The purified laccase had a blue color, typical of copper containing proteins. The UV-vis spectrum of the laccase (Fig. 8a) showed peak absorption at about 611 nm, typical for the type I Cu (II) that is responsible for the deep blue color of the enzyme. A shoulder at about 333 nm suggests the presence of the type III binuclear Cu (II) pair. Similar results were obtained by other researchers ([28]; [18]; [34]).
Fig. 8.
Laccase enzyme characterization, a) UV-Vis spectroscopy analysis, b) Thermal gram analysis (TGA), c) Differential scanning calorimetric (DSC) analysis of laccase enzyme shows sharp endothermic peak at 150 °C which corresponds to their melting point temperature range of 145-150 °C and d) FT-IR analysis.
The UV-vis spectrum of the purified laccase showed a peak of absorption at around 600 nm, typical for the type I Cu (II), which is responsible for the deep blue color of the enzyme. A shoulder at around 325 nm suggests the presence of the type III binuclear Cu (II) pair (Reinhammar, 1984). The spectral characteristics of laccase from Penicillium chrysogenum were similar to that observed for another laccases [25,34,35].
3.9. Thermal gravimetric analysis (TGA)
The sample was subjected to the TGA analysis, which shown the water loss at 100 °C where the weight loss was 20% meaning the moisture content of the sample was around 20%. The first step of degradation of the sample started at 270 °C to 450 °C where weight loss in this range was about 60% and the final residue obtained was around 13% meaning higher carbons present in the sample. The results are shown in Fig. 8b.
3.10. Differential scanning calorimetery (DSC) analysis of laccase enzyme
Differential scanning calorimetric (DSC) analysis is a useful tool to monitor the thermal behavior (melting/denaturing temperature) of laccase enzyme. The laccase enzyme was subjected to the DSC analysis shows sharp endothermic peak at 150 °C which corresponds to their melting point temperature range between 145-155 °C (Fig. 8c).
3.11. FT-IR analysis of purified laccase enzyme
The functions of laccase enzyme were depends on the functional group present in the active site of the enzyme protein and it is imperative to know the characteristics features of the enzyme. The FT-IR analysis was carried out for the laccase enzyme for finding out the functional group of the enzyme protein (Fig. 8d). Laccase enzyme contains three important functional group including the amino, carboxylic, and thiol group, which are present in the active site of the enzyme protein and which will react with the substrates. The broad band appear in the region of 3400 cm-1 is corresponding to the intermolecular hydrogen bonding, which is arising from the –NH2 and –OH groups in enzyme protein, which is indicating the presence of amide (amino acid groups). The band observed at the region of 1405 cm-1 and 1090 cm-1 are corresponding to the presence of functional group C = C stretching and C-N stretching, which is indicating the presence of the presence of Amide I band. In the FT-IR spectrum the band observed at the region of 2956 cm-1 corresponds to the functional group of OH/ or NH and 1656 cm-1 corresponds to functional group of C = O stretching, which is indicating the presence of Amide II band (carbonyl group) and also indicating the presence of copper site. The other researchers are also revealed the same features ([36]; [37]).
3.12. Redox potential
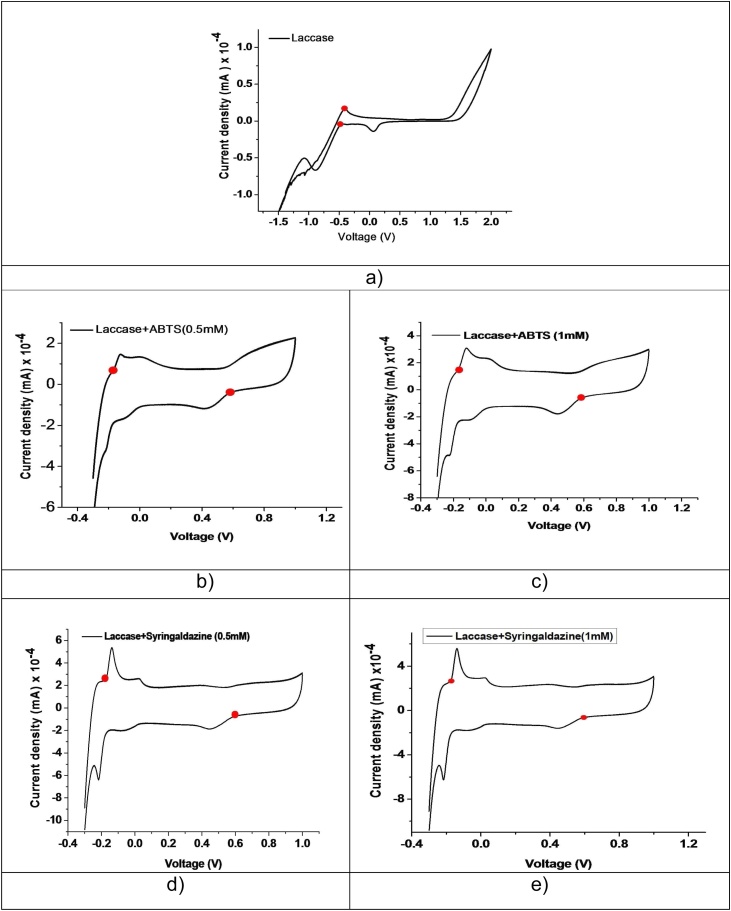
Laccases are oxidative group of multicopper protein enzyme. The corresponding oxidation potential of the laccase enzyme is depends on the redox potential, which is directly related to the primary structure of the protein as well as T1 active site. The laccase redox potential was classified in to three groups like low (430 mV), medium (ranges from 470 to 710 mV) and high (about 780 mV) which was similar to others findings [25,38]. The redox potential of the laccase is calculated by in terms of current, expressed in either volts or millivolts measured by cyclic voltammetry analysis by calculating the effective electron donors in the laccase-catalysed reduction of dioxygen to water [38,39].
In present study the laccase obtained from Penicillium chrysogenum was subjected to the analysis of redox potential using cyclic voltammetry and with two different substrates capable for mediating laccase catalysed reactions were analysed at the concentrations of 0.5 & 1 mM of ABTS and syringaldazine (Fig. 9). The experiment gave some interesting result which was observed from the cyclic votametry for the laccase enzyme and its two different substrates. From the study we observed that the low reduction potential values were obtained at 455 mV for parent laccase and there is no oxidation potential was observed (Fig. 9a). However, in the case of laccase enzyme by reaction with 0.5 mM ABTS substrates enhanced the reversible oxidation potential at 580 mV and simultaneously the potential of reduction was decreased at 179 mV (Fig. 9b). It is two fold lesser than that parent laccase enzyme. When the concentration is increased by the addition of 1 mM ABTS to the laccase enzyme, the observed reduction potential is 165 mV. The reduction potential was consistently decreased with increasing the concentration but in the case of oxidation potential was slightly enhanced 590 mV (Fig. 9c). It is known fact that this behavior is related to the reaction between ABTS and ABTS2+ to give the cation radical ABTS+ as reported earlier.
Fig. 9.
Cyclic voltametry analysis of laccase enzyme with different substrates (ABTS and Syringaldazine).
The corresponding laccase enzyme was added with 0.5 mM and 1 mM syringaldazine, the observed reduction potential for these concentrations is ˜175 mV. Interestingly for this case, the result of oxidation potential is higher than that of previous substrate (ABTS). The observed oxidation potential is 592 mV and 600 mV respectively for the concentration of 0.5 mM and 1 mM syringaldazine (Fig. 9d and e). The laccase is low redox potential enzyme, which need effective mediator to enhance the oxidation potential of the enzyme. So ABTS and syringaldazine were used as substrates to enhance the oxidation potential, which responded well and enhanced the oxidation potential of the laccase enzyme ([34]; [40]). The laccase enzyme contains type 1 copper (Cu+1) state, after adding substrates, which is converted into type 3 copper (Cu+3) state. It is clearly indicates that the mediator interacts with laccase very well [41].
3.13. Effect of pH and temperature on laccase activity
Either at lower (pH = 4.5) or at higher pH (pH = 6.5), enzymatic activity has been found to be low. Maximum laccase activity was obtained with 1.3 g/l of yeast extract and at pH 5.5. With increase in temperature, laccase activity is found to increase.
3.14. Effect of inhibitors on laccase activity
The inhibitory effect of EDTA, thioglycolic acid, sodium azide (NaN3), ethanol and diethyldithiocarbamic acid (DDC) on laccase activity was studied and the results are presented in Table 5. The water miscible ethanol solvent causes the decrease of laccase activity when its concentration exceed beyond 25% and thioglycolic acid decreases the laccase activity upto 90.7% at the concentration of 815 μm. The huge decrease of laccase activity was due to change in pH conditions in aqueous solution that produces the inhibition of enzyme activity [42]. Laccases are sensitive for metals even at low concentrations and inhibit the laccase activity. In this present study, the sodium azide was tested for the inhibition activity, which inhibited the laccase activity completely at the concentration of 15 μm. It was reported that the laccase loses its activity completely during the blocking of internal electron transfer reaction by the binding of sodium azide to the copper site (type 2 and type 3) in the laccase enzyme.
Table 5.
Effect of inhibitors on laccase activity
| Inhibitors | Concentration | % inhibition |
|---|---|---|
| Diethyldithiocarbamic acid (DDC) (mM) | 0.1 | 25.6 |
| 0.2 | 41.3 | |
| 0.5 | 64.8 | |
| 1.0 | 99.9 | |
| Ethylenediaminetetraacetic acid (EDTA) | 1 | 16.8 |
| 5 | 40.3 | |
| 10 | 62.8 | |
| 25 | 94.5 | |
| Ethanol (%) | 10 | 3.4 |
| 20 | 8.9 | |
| 50 | 48.9 | |
| 70 | 100 | |
| Sodium azide (NaN3) (μM) | 2 | 14.0 |
| 5 | 38.7 | |
| 10 | 69.2 | |
| 20 | 100 | |
| Thioglycolic acid (TGA) (μM) | 100 | 13.3 |
| 250 | 32.6 | |
| 500 | 54.1 | |
| 750 | 92.4 |
The laccase was mildly inhibited by the metal chelator EDTA (5 mM) and more strongly inhibited by the copper chelator diethyldithiocarbamic acid (DDC) at 1 mM concentration. Laccase activity was strongly inhibited by metal specific chelators rather than by general metal chelators [43].
3.15. Effect of metal ions on laccase activity
The interaction of metals with extracellular laccase was particularly important for the better understanding of the biotechnological processes of xenobiotic degradation. Therefore, the stability of laccase activity against several metal compounds was tested (Table 6). Metals such as Co, Sn, Hg, Fe, K, Zn, Mg, Mn, Na, Ba, Cr, and Ca were assessed. Metal ion concentration of 1 mM had no significant effect over laccase activity except Hg, which caused 17.2% inhibition. When the metal ion concentration was increased to 5 mM, Cr, Zn and Sn inhibited laccase activity by 13.8, 11.4 and 9.7%, respectively; whereas the laccase activity was highly sensitive to 5 mM Hg showing 25.4% inhibition, indicating the presence of thiol groups, essential for its activity. The purified laccase from the edible mushroom Lentinula edodes was inhibited in the presence of 1 mM Sn2+ (99%), Ca2+ (70%), Zn2+ (64%), Hg2+ (55%), K+ (54%), and Cd2+ (45%), and it was activated by 40% in the presence of 10 mM Cu2+. The activation of laccase by Cu2+ may be due to the filling of type-2 copper binding sites with copper ions [44]. The observations indicated that the effect of metal ions on laccase activity was highly dependent on its source and the type of metals used, which had a great influence on the catalytic activity of the enzyme. The activation or inhibition of proteolytic enzymes by metals could change the turnover rate of extracellular enzymes.
Table 6.
Effect of metal ions on laccase activity
| Metal ion | Concentration (mM) | % Inhibition |
|---|---|---|
| Cobalt | 1 | 1.8 |
| 5 | 3.6 | |
| Tin | 1 | 1.1 |
| 5 | 9.7 | |
| Mercury | 1 | 17.2 |
| 5 | 25.4 | |
| Ferric | 1 | 1.5 |
| 5 | 2.0 | |
| Potassium | 1 | 3.3 |
| 5 | 3.9 | |
| Zinc | 1 | 7.6 |
| 5 | 11.4 | |
| Magnesium | 1 | 1.4 |
| 5 | 2.8 | |
| Manganese | 1 | 0.8 |
| 5 | 1.2 | |
| Sodium | 1 | Nil |
| 5 | Nil | |
| Barium | 1 | Nil |
| 5 | 0.6 | |
| Chromium | 1 | 1.7 |
| 5 | 13.8 | |
| Calcium | 1 | 0.6 |
| 5 | 3.5 |
4. Conclusions
The extracellular laccase producing potential white-rot fungus was isolated from tree and used for enzyme production. Based on the screening results using various substrates of ABTS, guaiacol and tannic acid, white-rot fungus, identified by 18 s rRNA as Penicillium chrysogenum and its medium optimization by classical one-factor-at-a-time method and central composite rotatable design (CCRD) using a tool of response surface methodology (RSM). The optimum laccase activity of 6.0 U ml-1 was obtained by classical method and maximum activity of 7.9 U ml-1 was achieved by statistical optimization of the medium at 32 °C and 5 days. This activity was 2.46 times greater than the initial activity of 3.2 U ml-1 from the liquid culture medium.
The microbial strain was isolated from the tree at the premises of CSIR-CLRI, Chennai, Tamilnadu, India belonged to Penicillium chrysogenum fungal species known to produce laccase enzyme in optimized medium under submerged conditions using copper sulphate as inducer. The purified Penicillium chrysogenum fungus laccase is found to have molecular weight approximately 68 kDa. The UV-vis spectrum of the purified laccase showed a peak of absorption at around 608 nm, typical for the type I Cu (II), which is responsible for the deep blue color of the enzyme. A shoulder at around 325 nm suggests the presence of the type III binuclear Cu (II) pair. The spectral characteristics of laccase from Penicillium chrysogenum were similar to that observed for other fungus laccases in literature.
Conflict of interests
The authors declare that there is no conflict of interest.
Acknowledgements
One of the authors T. Senthilvelan thanks the University Grants Commission (UGC), New Delhi, India for the financial grant through research fellowship (JRF) in carrying out this work. On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Birhanli E., Yesilada O. Increased production of laccase by pellets of Funalia trogii ATCC 200800 and Trametes versicolor 200801 in repeated-batch mode. Enzyme Microbial Technology. 2006;39:1286–1293. [Google Scholar]
- 2.Chawla S., Rawal R., Shabnam Kuhad R.C., Pundir C.S. An amperometric polyphenol biosensor based on laccase immobilized on epoxy resin membrane. Anal. Methods. 2011;3:709–714. doi: 10.1039/c0ay00679c. [DOI] [PubMed] [Google Scholar]
- 3.Levin L., Forchiassin F., Ramos A.M. Copper induction of lignin-modifying enzymes in the white-rot fungus Trametes trogii. Mycologia. 2002;94:377–383. [PubMed] [Google Scholar]
- 4.Valeriano V.S., Silva A.M.F., Santiago M.F., Bara M.T.F., Garcia T.A. Production of laccase by Pycnoporus sanguineus using 2, 5-xylidine and ethanol. Brazilian Journal of Microbiology. 2009;40:790–794. doi: 10.1590/S1517-83822009000400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giardina P., Faraco V., Pezzella C., Piscitelli A., Vanhulle S., Sannia G. Laccases: a never-ending story. Cellular and Molecular Life Sciences. 2010;67:369–385. doi: 10.1007/s00018-009-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sivakumar R., Rajendran R., Balakumar C., Tamilvendan M. Isolation, screening and optimization of production medium for thermostable laccase production from Ganoderma sp. International journal of Engineering Science and Technology. 2010;2(12):7133–7141. [Google Scholar]
- 7.Arora D.S., Sharma R. Ligninolytic fungal laccase and their biotechnological applications. Appli. Biochem. Bitechnol. 2010;160:1760–1788. doi: 10.1007/s12010-009-8676-y. [DOI] [PubMed] [Google Scholar]
- 8.Couto S.R., Herrera J.L.T. Industrial and biotechnological applications of laccases: a review. Biotechnol. Advanc. 2006;24:500–513. doi: 10.1016/j.biotechadv.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Ferry Y., Leech D. Amperometric detection of catecholamine neurotransmitters using electrocatalytic substrate recycling at a laccase electrode. Electroanalysis. 2005;17:113–119. [Google Scholar]
- 10.Mustafa R., Muniglia L., Rovel B., Girardin M. Phenolic colorants obtained by enzymatic synthesis using a fungal laccase in a hydro-organic biphasic system. Food Research International. 2005;38:995–1000. [Google Scholar]
- 11.Minussi R.C., Pastore G.M., Duran N. Potential applications of laccase in the food industry. Trends in Food Science & Technology. 2002;13:205–216. [Google Scholar]
- 12.Yarpolov A.I., Skorobogat’ko O.V., Vartanov S.S., Varfolomeyev S.D. Laccase: properties, catalytic mechanism, and applicability. Applied Biochemistry and Biotechnology. 1994;49:257–280. [Google Scholar]
- 13.Mikolasch A., Schauer F. Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Applied Microbiology and Biotechnology. 2009;82:605–624. doi: 10.1007/s00253-009-1869-z. [DOI] [PubMed] [Google Scholar]
- 14.Saito T., Kato K., Yogogawa Y., Nishida M., Yamashita N. Detoxification of bisphenol A and nonylphenol by purified extracellular laccase from a fungus isolated from soil. Journal of Bioscience and Bioengineering. 2004;98:64–66. doi: 10.1016/S1389-1723(04)70243-1. [DOI] [PubMed] [Google Scholar]
- 15.Borbonnais R., Paice M.G., Freiermoth B., Bodie E., Borneman S. Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Applied Environmental Microbiology. 1997;63:4627–4632. doi: 10.1128/aem.63.12.4627-4632.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calcaterra A., Galli C., Gentili P. Journal of Molecular Catalysis B: Enzymatic. 2008;51:118–120. [Google Scholar]
- 17.Husain M., Husain Q. Critical Reviews in Environmental Science and Technology. 2008;38:1–42. [Google Scholar]
- 18.Mishra A., Kumar S., Pandey A.K. Laccase production and simultaneous decolorisation of synthetic dyes in unique inexpensive medium by new isolates of white rot fungus. International Biodeterioration and Biodegradation. 2011;65:487–493. [Google Scholar]
- 19.Birhanli E., Erdogan Selim, Yesiladaa Ozfer, Onal Yunus. Laccase production by newly isolated white rot fungus Funalia trogii: Effect of immobilization matrix on laccase production. Biochemical Engineering Journal. 2013;71:134–139. [Google Scholar]
- 20.Mathur G., Mathur A., Sharma B.M., Chauhan R.S. Enhanced production of laccase from Coriolus sp. using Plackett-Burman design. Journal of Pharmacy Reseach. 2013;6:151–154. [Google Scholar]
- 21.Vijayaraghavan P., Vincent S.G.P. Medium optimization for the production of fibrinolytic enzyme by Paenibacillus sp. IND8 using response surface methodology. The Scientific World Journal. 2014;014 doi: 10.1155/2014/276942. Hindawi Publishing Corporation. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neifar Mohamed, Kamoun Amel, Jaouani Atef, Ellouze-Ghorbel Raoudha, Ellouze-Chaabouni Semia. Application of Asymetrical and Hoke Designs for Optimization of Laccase Production by the White-Rot Fungus Fomes fomentarius in Solid-State Fermentation. Enzyme Research. 2011 doi: 10.4061/2011/368525. 1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niladevi K.N., Prema P. Effect of inducers and process parameters on laccase production by Streptomyces psammoticus and its application in dye decolorization. Bioresource Technology. 2008;99:4583–4589. doi: 10.1016/j.biortech.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 24.El-Zayat S.A. Preliminary studies on laccase production by Chaetomium globosum an endophytic fungus in Glinus lotoides. American-Eurasian J. Agric. & Environ. Sci. 2008;1:86–90. 3. [Google Scholar]
- 25.Sadhasivam S., Savitha S., Swaminathan K., Lin F.-H. Production, purification and characterization of mid-redox potential laccase from a newly isolated Trichoderma harzianum WL1. Proc Biochem. 2008;43:736–742. [Google Scholar]
- 26.Nicolini C., Bruzzese D., Cambria M.T., Bragazzi N.L., Pechkova E. Recombinant laccase: I. Enzyme cloning and characterization. Journal of cellular biochemistry. 2013;114:599–605. doi: 10.1002/jcb.24397. [DOI] [PubMed] [Google Scholar]
- 27.Ramesh Rajendran, Aarthy Mayilvahanan, Kuppuswami Marichetti, Gowthaman Katya, Gabrovska Tzonka, Godjevargova, Kamini Numbi Ramudu. Screening and production of a potent extracellular Arthrobacter creatinolyticus urease for determination of heavy metal ions. J. Basic Microbiol. 2014;54:285–295. doi: 10.1002/jobm.201200561. [DOI] [PubMed] [Google Scholar]
- 28.Eggert C., Temp U., Eriksson K.-E.L. The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl. Environ. Microbiol. 1996;62:1151–1158. doi: 10.1128/aem.62.4.1151-1158.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chefetz B., Chen Y., Hader Y. Purification and characterization of laccase from Chaetomium thermophilium and its role in humification. Appl. Environ. Microbiol. 1998;64:3175–3179. doi: 10.1128/aem.64.9.3175-3179.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peter M.G., Wollenberger U. Phenol-oxidizing enzymes: mechanisms and applications in biosensors. In: Scheller F.W., Schubert F., Fedrowitz J., editors. vol. 1. Birkhauser; Basel: 1997. pp. 63–82. (Frontiers in biosensorics). EXS 80. [DOI] [PubMed] [Google Scholar]
- 31.Xu F. Laccase. In: Flickinger M.C., Drew S.W., editors. Encyclopedia of bioprocess technology: Fermentation, biocatalysis, bioseparation. John Wiley & Sons Inc.; New York: 1999. pp. 1545–1554. [Google Scholar]
- 32.Baldrian P. Fungal laccases occurrence and properties. FEMS Microb. Rev. 2006;30(2):215–242. doi: 10.1111/j.1574-4976.2005.00010.x. [DOI] [PubMed] [Google Scholar]
- 33.Madhavi V., Lele S.S. Laccase: Properties and applications. Bioresorces. 2009;4(4):1694–1717. [Google Scholar]
- 34.Shleev S.V., Morozova O.V., Nikitina O.V., Gorshina E.S., Rusinova T.V., Serezhenkov V.A. Comparison of physic-chemical characteristics of four laccases from different basidiomycetes. Biochemie. 2004;86:693–703. doi: 10.1016/j.biochi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Palonen H., Saloheimo M., Viikari L., Kruus K. Purification, characterization and sequence analysis of a laccase from the ascomycete Mauginiella sp. Enzyme Microb. Technol. 2003;33:854–862. [Google Scholar]
- 36.Gustiananda M., Haris Parvez I., Milburn Peter J., Gready Jill E. Copper-induced conformational change in a marsupial prion protein repeat peptide probed using FTIR spectroscopy. FEBS Letters. 2002;512:38–42. doi: 10.1016/s0014-5793(01)03298-7. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed I. El-Batal, ElKenawy Nora M., Yassin Aymen S., Amin Magdy A. Laccase production by Pleurotus ostreatus and its application in synthesis of gold nanoparticles. Biotechnology Reports. 2015;5:31–39. doi: 10.1016/j.btre.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reinhammar B.R.M. Oxidation-reduction potentials of the electron acceptors in laccases and stellacyanin. Biochim Biophys Acta. 1972;275:245–259. doi: 10.1016/0005-2728(72)90045-x. [DOI] [PubMed] [Google Scholar]
- 39.Xu F., Shin W., Brown S.H., Wahleitner J.A., Sundaram U.M., Solomon E.I. A study of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity and stability. Biochim Biophys Acta. 1996;1292:303–311. doi: 10.1016/0167-4838(95)00210-3. [DOI] [PubMed] [Google Scholar]
- 40.Xu F., Berka R.M., Wahleithner J.A., Nelson B.A., Shuster J.R., Brown S.H. Site-directed mutations in fungal laccase: effect on redox potential activity and pH profile. Biochem J. 1998;334:64–70. doi: 10.1042/bj3340063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jayalakshmi M., Balasubramanian K. Cyclic Voltammetric Behavior of Copper Powder Immobilized on Paraffin Impregnated Graphite Electrode in Dilute Alkali Solution. Int. J. Electrochem. Sci. 2008;3:1277–1287. [Google Scholar]
- 42.Rodakiewicz-Nowak J., Kasture S.M., Dudek B., Haber J. Effect of various water miscible solvents on enzymatic activity of fungal laccases. J. Mol. Catal. B-Enzym. 2002;11:1–11. [Google Scholar]
- 43.Ryan S., Schnitzhufer W., Tzanov T., Cavaco-Paulo A., Gubitz G.M. An acid-stable laccase from Sclerotium rolfsii with potential for wood dye decolourization. Enzyme Microb. Technol. 2003;33:766–774. [Google Scholar]
- 44.Nagai M., Sato T., Watanable H., Saito K., Kawata M., Enei H. Purification and characterization of an extracellular laccase from the edible mushroom Lentinula edodes, and decolorization of chemically different dyes. Appl. Microbiol. Technol. 2002;60:327–335. doi: 10.1007/s00253-002-1109-2. [DOI] [PubMed] [Google Scholar]