Abstract
Obstructive sleep apnea (OSA) is a common disease in adults, which influences human relations, quality of life and associates with major complications. Continuous positive airway pressure (CPAP) is the gold standard treatment modality in OSA patients. For patients incompliant or unwilling to CPAP therapy, surgery is an alternative treatment. Sleep surgery for OSA include intrapharyngeal surgery, extrapharyngeal surgery and bariatric surgery addressing upper airway soft tissue, maxillofacial bone, and obesity, respectively. Among sleep surgeries, intrapharyngeal surgery (soft tissue surgery) is widespread used and serves overwhelming majority in OSA surgical patients. Despite the popularity of intrapharyngeal surgery, its outcomes can be influenced by multiple factors and consequently need conjunctive remedy to enhance at the short-term and sustain in the long-term. In this article, we introduce updated indications for treating OSA, practical principle in decision-making between CPAP and surgery, hybrid procedures in treating obstruction at the nose, palate, tongue and epiglottis, and postoperative integrated treatment including oropharyngeal myofunctional therapy (local), positional therapy (regional), and body weight reduction (systemic), and circadian rhythm (central). In summary, intrapharyngeal surgery is a target-oriented procedure that needs to be performed precisely and combines with integrated treatment as a holistic care for OSA patients.
Keywords: Obstructive sleep apnea, Intrapharyngeal surgery, Mini-invasive septoturbinoplasty, Suspension palatoplasty, Hybrid treatment
Updated indications for treating OSA
Traditional indications for treating obstructive sleep apnea (OSA) are to improve clinical symptoms (mainly in snoring and daytime sleepiness) and reduce major complications (hypertension, myocardial infarction, stroke, sudden death) [1], [2], [3]. Recently, robust evidences show OSA patients have higher prevalence of vertigo, tinnitus and sudden deafness than non-OSA people and repeated nocturnal hypoxemia may be the link between OSA and inner ear disease but warrants further research to confirm [4], [5], [6], [7], [8]. Preliminary reports showed that continuous positive airway pressure (CPAP) therapy improved both OSA and inner ear symptoms at the same time [6], [9]. Consequently, vertigo, tinnitus and sudden deafness may be additional indications for the treatment of OSA.
Principle in decision-making between CPAP and surgery
Decision-making for OSA by CPAP or surgery is always debating because of the uncertainty in the disease character-anatomical or neurological disease [10]. Anatomical anomaly is clearly noted in some patients, however, grossly normal in the physical examination is found in others. In general, OSA is a disease with entity of both anatomical deformity and neurological deficiency, which are different in the proportion in individual OSA patients [11].
The introducing of obstruction/collapse concept can be helpful to elucidate the confusion and facilitate decision-making of treatment plan. There are obviously different between “obstruction” and “collapse” in different sleep status and positions. Obstruction reflects anatomical deformity that can be shown in routine physical examination, cephalometry, and awake fiberoptic nasopharyngoscopy, such as septal deviation, tonsillar hypertrophy, long uvula, webbing of the posterior pillar, lingual tonsillar hypertrophy, long epiglottis, etc. Obstructive lesions do not have significant change between awake/sleep status and supine/lateral position, and usually have clear anatomical demarcation. By contrast, collapse always implies neurological deficiency that is relative “gross normal” without clear demarcation of lesion and becomes narrowing only in sleep and particular position (mainly supine position) such as tongue and lateral pharyngeal wall collapse [12]. Obstruction and collapse are not mutually exclusive. OSA patients may have both obstruction and collapse with different proportion in individual patients such as tongue collapse with lingual tonsil hypertrophy, and soft palate collapse with palatal tonsillar hypertrophy.
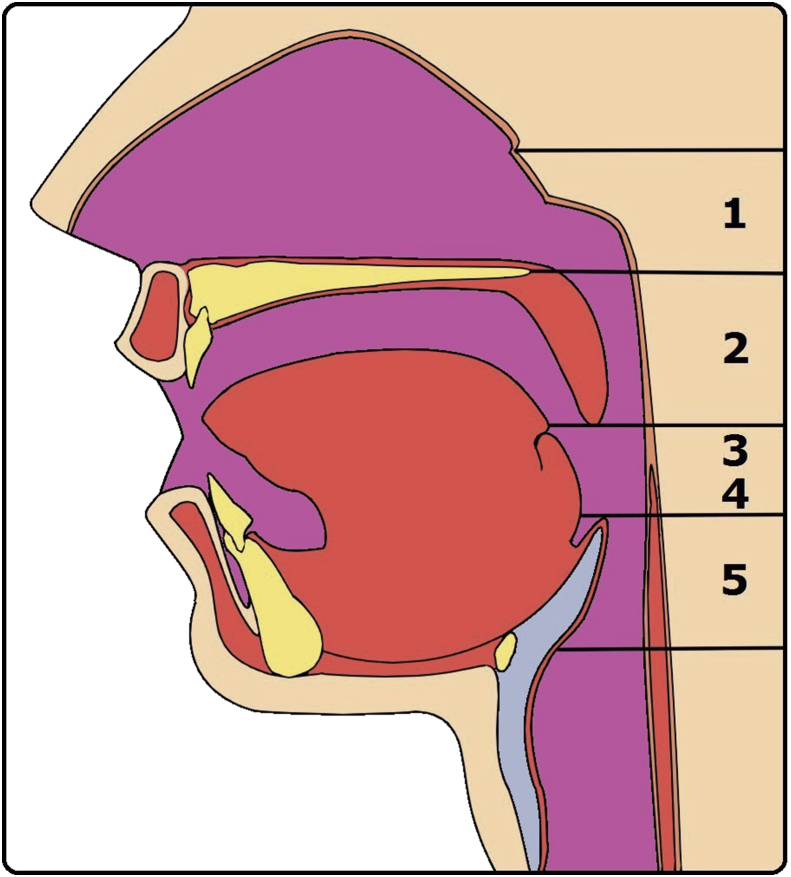
Surgery is best implemented in the obstruction because of well demonstration of the lesion with clear surgical margin in routine daytime examination [11]. On the contrary; CPAP has superiority in splinting airway collapse, which has no definite lesion and clear margin during awake examination [13], [14]. Two exceptions in the treatment of airway collapse by CPAP are nasal valve and epiglottis collapses, in which CPAP does not have optimal response and surgery commonly lead to improvement in clinical symptoms and OSA severity [15], [16], [17]. Based on the widely used nose-VOTE system, surgery takes advantage in treating obstruction/collapse of the nose, velopharynx, and epiglottis [18], [19], [20], by contrast, CPAP has superiority in treating collapse from the oropharyngeal lateral wall and tongue [21]. Fig. 1 demonstrates the treatment strategy in individual level of the upper airway. It's also suggested for sleep specialist to exclude airway obstruction and nasal/epiglottic collapse before prescribing CPAP for OSA patients to improve its compliance.
Fig. 1.
Superiority of treatment modality in nose-VOTE system. Surgery takes advantage in the nose (1), velopharynx (2), and epiglottis (5). CPAP has advantage in the lateral pharyngeal wall (3) and tongue (4).
OSA is a multifactorial disease that needs multi-disciplinary approach and consequently multi-modality treatment. CPAP and surgery are not exclusive from each other; actually, they can have mutual benefits and reciprocity. For example, nasal, tongue base and multiple-level surgery can reduce CPAP pressure and improve CPAP compliance in OSA [22], [23], [24]. By contrast, CPAP can be used to stabilize airway after intrapharyngeal surgery especially in tongue surgery [25].
Intrapharyngeal surgery by mini-invasive reconstruction
Sleep surgery can be multi-dimensional to involve local, regional or systemic; local-soft tissue (Intra-pharyngeal) surgery, regional-skeletal (extrapharyngeal) surgery, and systemic-bariatric surgery [26], [27], [28], [29]. OSA patients with craniofacial anomaly (such as retro/micrognathia) or narrowing of hard palate are exclusively suggested to skeletal surgery. In the meanwhile, OSA patients with pathological obesity are referred to bariatric surgery [30]. Treatment of craniofacial anomaly or pathological obesity usually takes precedence over soft tissue surgery because of concerns in safety and efficacy.
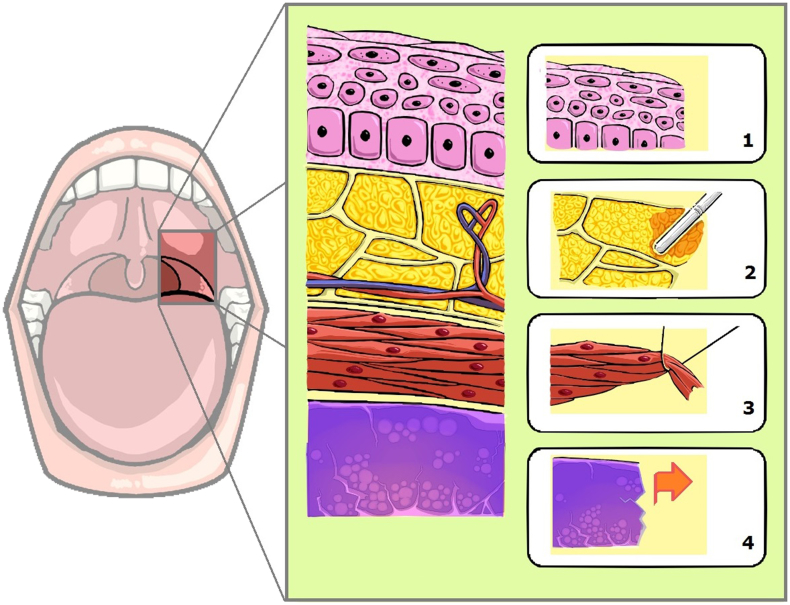
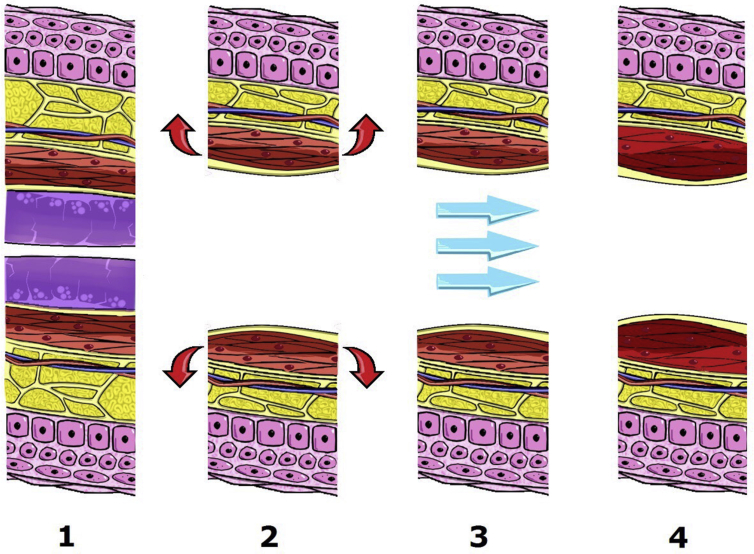
Intra-pharyngeal surgery for OSA is a soft tissue-targeted surgery that can involve the nose, soft palate, lateral pharyngeal wall, tongue and epiglottis. Surgical technique of intra-pharyngeal surgery for OSA has transformed from radical excision of “redundant” soft tissue for the enlargement of airway toward mini-invasive reconstruction to fulfill both preservation of pharyngeal function and improvement in sleep apnea. The term of mini-invasive reconstruction at intra-pharyngeal surgery is not only a concept or technique but can be implemented practically into histological gradation: (1) mucosa: preservation, (2) adipose tissue: ablation, (3) lymphoid tissue: excision, (4) muscle: suspension (Fig. 2). Based on the principle, intra-pharyngeal surgery involves mixed techniques in individual level of airway obstruction. Table 1 shows the hybrid of 3 techniques (excision, suspension and ablation) in nose-VOTE system.
Fig. 2.
Hybrid reconstruction of intra-pharyngeal surgery is implemented into histological gradation: mucosa: preservation (1), adipose tissue: ablation (2), muscle: suspension (3), lymphoid tissue: excision (4).
Table 1.
The use of hybrid reconstruction (excision, suspension and ablation) in nose-VOTE system.
| Excision | Suspension | Ablation | |
|---|---|---|---|
| Nose | Deviated septum | Nasal valve | Turbinate |
| Velopharynx | Tonsil | Muscle | Adipose tissue |
| Tongue | Lingual tonsil | Tongue base | Lingual tissue |
| Epiglottis | cartilage | hyoid | – |
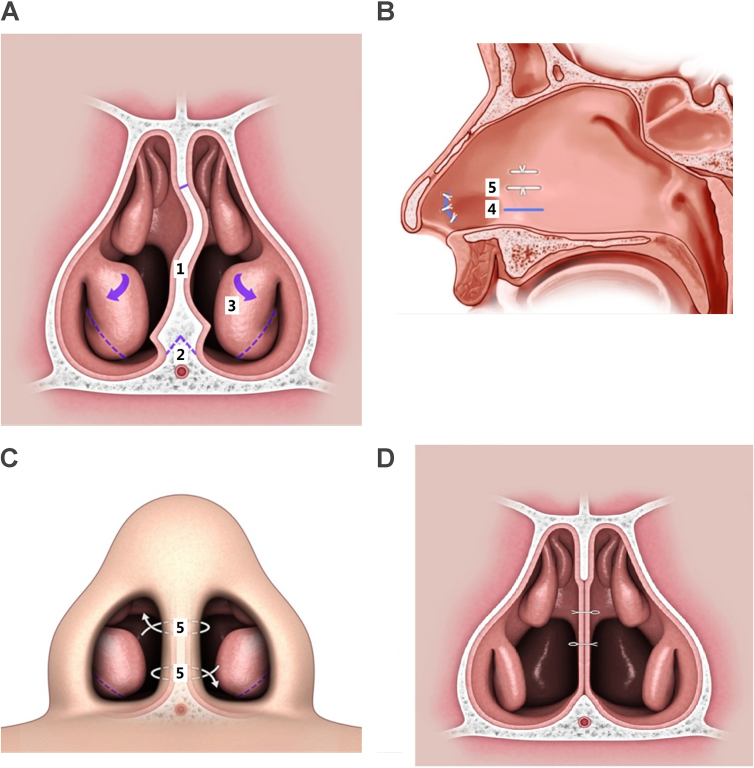
Nasal surgery is a common surgery for snoring and is a part of multi-level surgery for OSA. The procedure of nasal surgery has evolutionary changes toward mini-invasiveness to decrease the risk of septal hematoma and preserve the mucosal function of turbinate. Mini-invasive septoturbinoplasty (MIST) involves excision of deviated septum, triangular hump preservation of nasal floor to protect great palatine artery, incision drainage of bottom layer and trans-septal sutures to avoid septal hematoma, and out-fracture of inferior turbinate (Fig. 3). The MIST can effectively enlarge nasal passage, preserve nasal function, and prevent empty nose syndrome [18], [31], [32]. Previous studies revealed that nasal surgery improves snoring, daytime sleepiness, quality of life, and the use of CPAP or mandibular advancement device (MAD) [18], [31], [32]. Nasal surgery can also be used in multi-level surgery for OSA to enhance the surgical outcomes [33]. However, nasal surgery alone does not improve the severity of OSA in terms of apnea-hypopnea index (AHI) value [34], [35]. Good candidate of nasal surgery for snoring reduction is OSA patient with small tonsils (tonsil size I) [31]; by contrast, Good candidate of nasal surgery for OSA is patient with low tongue position (MMP I-II) [34]. In 5–10% of OSA patients, snoring exacerbated after nasal surgery [36]. The reason could be attributable to the change of sleep position from lateral to supine sleep because of improved nasal breathing. In small part of OSA patients, the improvement of OSA after nasal surgery may be attributed to the change of respiratory model from oral to nasal breathing in conjunction with minor pharyngeal obstruction and significant nasal obstruction [34].
Fig. 3.
Mini-invasive septoturbinoplasty involves (1) excision of deviated septum, (2) triangular hump preservation of nasal floor to protect great palatine artery and (3) out-fracture of inferior turbinate (A), incision drainage following nasal floor (4) and lateral view of trans-septal suture (5) to avoid septal hematoma (B), anterior view of trans-septal suture (C), postoperative picture (D).
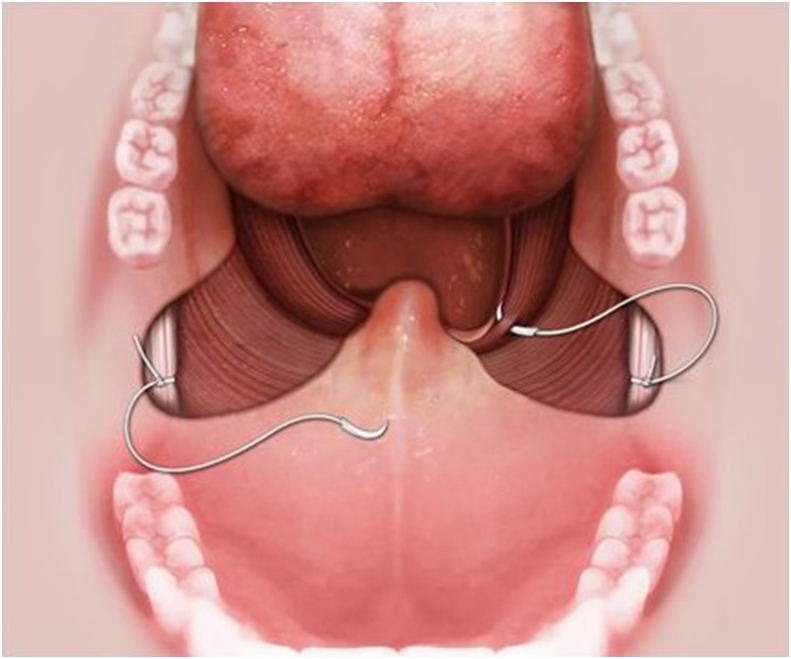
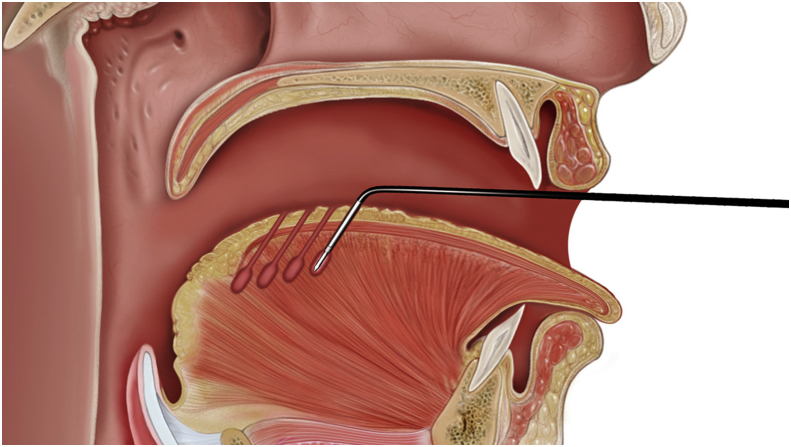
The use of suspension technique to substitute radical excision of soft palate in UPPP has been widely used in recent years [19], [37], [38]. The purpose of suspension in UPPP is to enlarge the velopharyngeal space for respiration, moreover, with no damage to normal pharyngeal function in phonation and swallowing [39]. The model of suspension is based on individual direction of velopharyngeal collapse during Muller's maneuver or drug-induced sleep endoscopy [40]. Lateral or relocation pharyngoplasty and suspension palatoplasty are suggested for lateral and anterior-posterior collapse of the velopharynx [19], [37], [38]. Relocation pharyngoplasty splints the superior pharyngeal muscle in order to ameliorate OSA from lateral pharyngeal wall collapse [38]. Suspension palatoplasty fixes the palatopharyngeus muscle to pterygomandibular raphe in order to advance the soft palate anteriorly [19]. (Fig. 4) For concentric collapse, a combined anterior-posterior and lateral suspension is needed to comprehensive improvement of the collapse from all directions. Previous studies showed no velopharyngeal insufficiency in terms of changes in voice, articulation and nasality after aforementioned surgeries [39]. We presumed those surgeries not only advance but also elevate soft palate that facilitate the contraction of levator veli palatinus muscle to separate the nasopharynx and oropharynx during swallowing and phonation [39]. Surgical results are superior to traditional UPPP in the reduction of sleep apnea [19], [37], [38], [41].
Fig. 4.
Suspension palatoplasty fixes the palatopharyngeus muscle to pterygomandibular raphe for anterior advancement of the soft palate.
OSA patients with tongue collapse can be treated by CPAP as the first priority and salvaged by surgery. Tongue base obstruction from lingual tonsil hypertrophy (LTH) grade III or IV can be excised through trans-oral robotic surgery or coblation endoscopic lingual lightening [42], [43], [44]. By contrast, small and collapsible tongue with retrognathia can be treated successfully through Repose tongue base suspension. Fig. 5 demonstrates widening of retroglossal space after Repose tongue base suspension in lateral cephalometry and fiberoptic laryngoscopy in one patient. Tongue body obstruction can be treated by coblation tissue volume reduction through multiple channels (Fig. 6). Outcomes of tongue surgery for OSA are difficult to determine in simultaneous multi-level surgery and highly variable in previous study [45]. The cause can be attributable to inappropriate mode or incorrect position. In our previous study of drug-induced sleep computed tomography scan in recognizing the levels of obstruction, we found that tongue collapse can be identified as upper (body, 30%), lower (base, 37%), and upper followed by lower (biphasic, 33%) collapse models from dynamic change in sagittal view [46]. Upper and biphasic models comprise 63% in our study population with tongue collapse, which are likely to be misdiagnosed as A-P collapse of the soft palate in drug-induced sleep endoscopy [47]. This can partially explain poor surgical outcomes of UPPP and variable tongue base surgery based on finding of drug-induced sleep endoscopy. Accordingly, the treatment of tongue collapse needs to consider from the viewpoint of whole tongue instead of tongue base only.
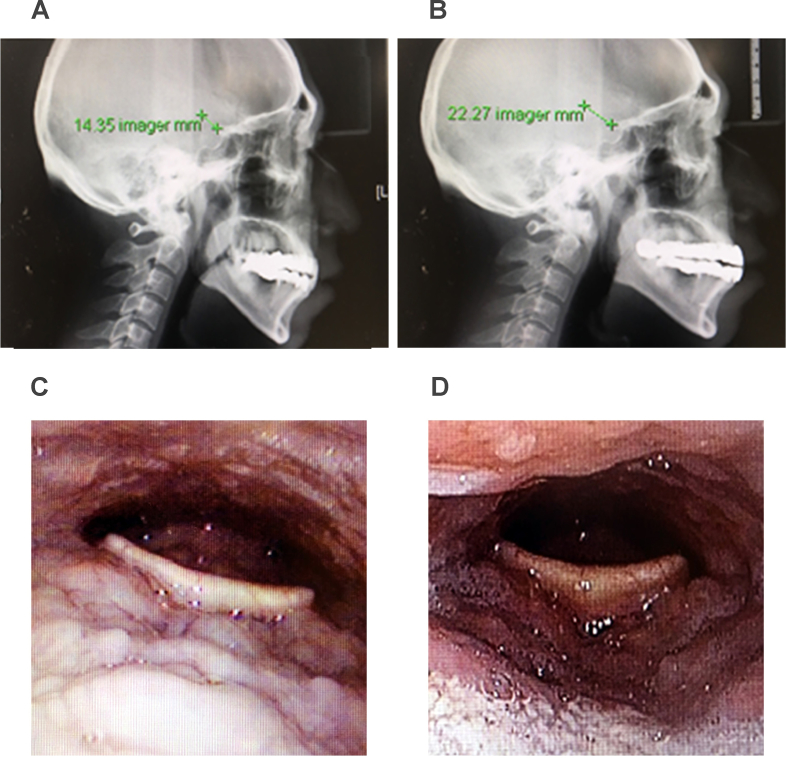
Fig. 5.
Perioperative change of retroglossal space following Repose tongue base suspension in lateral cephalometry (A-preoperative, B-postoperative) and fiberoptic laryngoscopy (C-preoperative, D-postoperative) in one patient.
Fig. 6.
Tongue body obstruction can be treated by coblation tissue volume reduction through multiple channels.
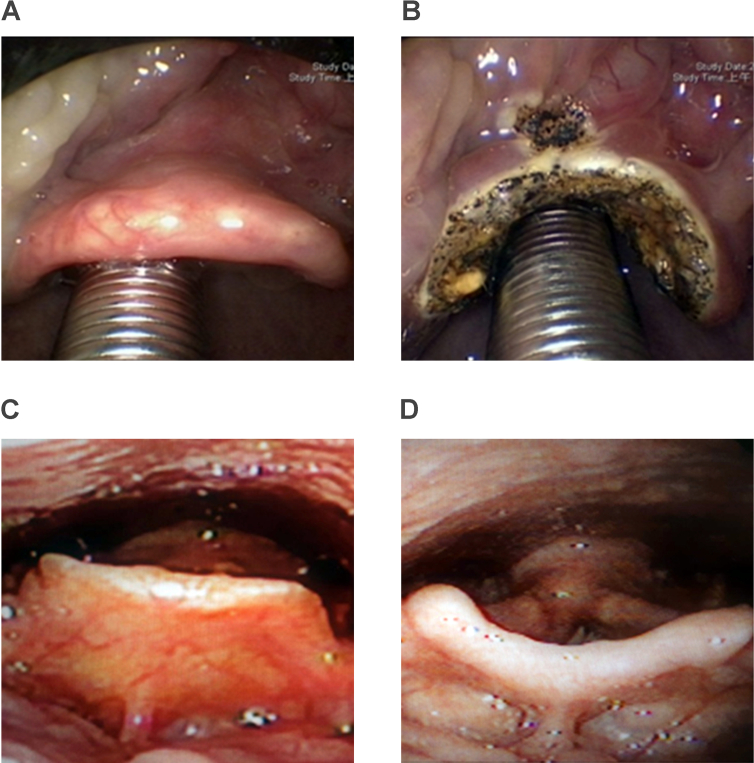
Epiglottis collapse is commonly ignored at its role in contributing to OSA. In our previous study, epiglottis collapse existed in 25% of moderate to severe OSA patients [46]. Epiglottis collapse jeopardizes the use of CPAP and causes UPPP failure [16], [17]. Partial epiglottidectomy is helpful to ameliorate the epiglottic collapse and consequently improve OSA [20]. (Fig. 7).
Fig. 7.
Perioperative change following partial horizontal epiglottidectomy for epiglottis collapse in OSA patient.
Postoperative integrated treatment
The outcomes of intrapharyngeal surgery can be influenced by many factors including stay-up, mouth breathing, sleep position, and obesity etc. [48] Furthermore, surgical outcomes commonly decline with time because of maturity of operation scar, relaxation of tightening procedure, and weakening of muscle tone that lead to a relapse in vibration and collapse of the upper airway during sleep [19]. Therefore, the treatment of OSA needs a comprehensive algorithm from reconstruction of the airway, restoration of the airflow to re-education of the muscles to enhance and sustain the outcomes after surgery (Fig. 8). The integrated treatment involves oropharyngeal myofunctional therapy (MFT) (local), positional therapy (regional), body weight reduction (systemic), and cognitive behavior therapy (central).
Fig. 8.
Integrated treatment of obstructive airway (1) obstructive airway before treatment, (2) reconstruction of airway, (3) restoration of airflow, and (4) re-education of muscle.
Oropharyngeal MFT includes oropharyngeal muscle exercise, posture and respiration training [49]. MFT can be used as preliminary therapy in mild OSA patients [50], conjunctive treatment after operation [49], and salvage management in recurrent surgical patients [51]. Theoretically, MFT to exercise the muscle in OSA patient is effective for airway “collapse”. By contrast, OSA patient with airway “obstruction” is not initially suggested for MFT unless the obstruction is relieved. MFT is best implemented in middle aged, non-obese, mild/moderate OSA patients [52]. The procedure can involve nasal breathing, respiration training, and the core value-muscle exercise in the soft palate, tongue, lateral pharyngeal wall, temporomandibular joint, face and neck [53]. The performance of MFT is composed of mode (isotonic/isometric), intensity (progressive usually), frequency (2–3/week commonly) and duration (3 months/course generally) [54]. The training of tongue muscle plays the most important role in MFT and its effect is not only to ameliorate tongue collapse but also to maintain adequate tongue position to avoid mouth breathing [55]. Studies showed oropharyngeal exercises improved sleep apnea and neck circumference on patients with moderate OSA [56].
The majority of OSA patients are supine position-dependent, this condition is particularly common in mild/moderate and non-obese OSA patients [56]. Accordingly positional therapy (lateral sleep) is helpful in these patients to resist the collapse and improve apnea [57]. Study revealed that positional therapy in positional OSA patients reshape the velopharyngeal airway, decreased its collapsibility during Muller maneuver, reduction of snoring intensity, and increase of oxygen saturation [58]. Further, the majority of non-positional OSA patients after palatal surgery turn out to be positional OSA patients [59]. This result rationalizes the necessity of positional therapy as a conjunctive treatment to enhance the UPPP outcomes.
Obesity can induce and interact with OSA through multiple mechanisms [60]. Loss of body weight can improve snoring and sleep apnea, by contrast, gain of body weight is a common factor in the recurrence of snoring and exacerbation of OSA after treatment [61]. Essentially, OSA patients with pathological obesity should be treated by CPAP and aggressive slimming such as bariatric surgery before intrapharyngeal surgery even patients with tonsillar hypertrophy [60]. By contrast, consultation of a dietitian for adequate controlling of the body weight is necessary for postoperative overweight OSA patients to enhance surgical outcomes and decrease recurrence of snoring and OSA [62].
Circadian rhythm and insomnia is associate the development of sleep-disordered breathing [63]. Stay-up and insomnia leads to reduced muscle tone and increased snoring and sleep apnea [64]. Consequently, a sleep hygiene education including early sleep is suggested after intra-pharyngeal surgery. Besides, some OSA patients need cognitive behavior therapy to improve comorbid insomnia and enhance surgical outcomes for the overlapping disease [65].
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgement
This project was supported by grants from Chang Gung Medical Foundation, Taoyuan, Taiwan (CORPG1F0071, CORPG3F0851 and IRB 201601393B0). The authors would like to thank Mr. Cheng-Shian Tsai for the illustrations and Ms Hsin-Chiao Lee, Ms Shu-Ting Liao, Ms Yen-Ting Chiang for documents collection.
Footnotes
Peer review under responsibility of Chang Gung University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bj.2019.02.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Yaggi H.K., Concato J., Kernan W.N., Lichtman J.H., Brass L.M., Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 2.Bradley T.D., Floras J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb D.J., Yenokyan G., Newman A.B., O'Connor G.T., Punjabi N.M., Quan S.F. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai M.S., Lee L.A., Tsai Y.T., Yang Y.H., Liu C.Y., Lin M.H. Sleep apnea and risk of vertigo: a nationwide population-based cohort study. Laryngoscope. 2018;128:763–768. doi: 10.1002/lary.26789. [DOI] [PubMed] [Google Scholar]
- 5.Sheu J.J., Wu C.S., Lin H.C. Association between obstructive sleep apnea and sudden sensorineural hearing loss. Arch Otolaryngol Head Neck Surg. 2012;138:55–59. doi: 10.1001/archoto.2011.227. [DOI] [PubMed] [Google Scholar]
- 6.Lai J.T., Shen P.H., Lin C.Y., Liu C.L., Liu T.C. Higher prevalence and increased severity of sleep-disordered breathing in male patients with chronic tinnitus: our experience with 173 cases. Clin Otolaryngol. 2018;43:722–725. doi: 10.1111/coa.13024. [DOI] [PubMed] [Google Scholar]
- 7.Kayabasi S., Iriz A., Cayonu M., Cengiz B., Acar A., Boynuegri S. Vestibular functions were found to be impaired in patients with moderate-to-severe obstructive sleep apnea. Laryngoscope. 2015;125:1244–1248. doi: 10.1002/lary.25021. [DOI] [PubMed] [Google Scholar]
- 8.Fischer Y., Yakinthou A., Mann W.J. Prevalence of obstructive sleep apnea syndrome (OSA) in patients with sudden hearing loss. A pilot study. HNO. 2003;51:462–466. doi: 10.1007/s00106-002-0712-y. [DOI] [PubMed] [Google Scholar]
- 9.Nakayama M., Masuda A., Ando K.B., Arima S., Kabaya K., Inagaki A. A pilot study on the efficacy of continuous positive airway pressure on the manifestations of Ménière's disease in patients with concomitant obstructive sleep apnea syndrome. J Clin Sleep Med. 2015;11:1101–1107. doi: 10.5664/jcsm.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broderick M., Guilleminault C. Neurological aspects of obstructive sleep apnea. Ann N Y Acad Sci. 2008;1142:44–57. doi: 10.1196/annals.1444.003. [DOI] [PubMed] [Google Scholar]
- 11.Li H.Y., Wang P.C., Lee L.A., Chen N.H., Fang T.J. Prediction of uvulopalatopharyngoplasty outcome: anatomy-based staging system versus severity-based staging system. Sleep. 2006;29:1537–1541. doi: 10.1093/sleep/29.12.1537. [DOI] [PubMed] [Google Scholar]
- 12.Genta P.R., Sands S.A., Butler J.P., Loring S.H., Katz E.S., Demko B.G. Airflow shape is associated with the pharyngeal structure causing OSA. Chest. 2017;152:537–546. doi: 10.1016/j.chest.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan C.E., Issa F.G., Berthon-Jones M., Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;317:862–865. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 14.Loube D.I., Gay P.C., Strohl K.P., Pack A.I., White D.P., Collop N.A. Indications for positive airway pressure treatment of adult obstructive sleep apnea patients: a consensus statement. Chest. 1999;115:863–866. doi: 10.1378/chest.115.3.863. [DOI] [PubMed] [Google Scholar]
- 15.Shimohata T., Shinoda H., Nakayama H., Ozawa T., Terajima K., Yoshizawa H. Daytime hypoxemia, sleep-disordered breathing, and laryngopharyngeal findings in multiple system atrophy. Arch Neurol. 2007;64:856–861. doi: 10.1001/archneur.64.6.856. [DOI] [PubMed] [Google Scholar]
- 16.Dedhia R.C., Rosen C.A., Soose R.J. What is the role of the larynx in adult obstructive sleep apnea? Laryngoscope. 2014;124:1029–1034. doi: 10.1002/lary.24494. [DOI] [PubMed] [Google Scholar]
- 17.Torre C., Camacho M., Liu S.Y., Huon L.K., Capasso R. Epiglottis collapse in adult obstructive sleep apnea: a systematic review. Laryngoscope. 2016;126:515–523. doi: 10.1002/lary.25589. [DOI] [PubMed] [Google Scholar]
- 18.Li H.Y., Lin Y., Chen N.H., Lee L.A., Fang T.J., Wang P.C. Improvement in quality of life after nasal surgery alone for patients with obstructive sleep apnea and nasal obstruction. Arch Otolaryngol Head Neck Surg. 2008;134:429–433. doi: 10.1001/archotol.134.4.429. [DOI] [PubMed] [Google Scholar]
- 19.Li H.Y., Lee L.A., Kezirian E.J., Nakayama M. Suspension palatoplasty for obstructive sleep apnea- a preliminary study. Sci Rep. 2018;8:4224. doi: 10.1038/s41598-018-22710-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golz A., Goldenberg D., Westerman S.T., Catalfumo F.J., Netzer A., Westerman L.M. Laser partial epiglottidectomy as a treatment for obstructive sleep apnea and laryngomalacia. Ann Otol Rhinol Laryngol. 2000;109:1140–1145. doi: 10.1177/000348940010901211. [DOI] [PubMed] [Google Scholar]
- 21.Torre C., Liu S.Y., Kushida C.A., Nekhendzy V., Huon L.K., Capasso R. Impact of continuous positive airway pressure in patients with obstructive sleep apnea during drug-induced sleep endoscopy. Clin Otolaryngol. 2017;42:1218–1223. doi: 10.1111/coa.12851. [DOI] [PubMed] [Google Scholar]
- 22.Poirier J., George C., Rotenberg B. The effect of nasal surgery on nasal continuous positive airway pressure compliance. Laryngoscope. 2014;124:317–319. doi: 10.1002/lary.24131. [DOI] [PubMed] [Google Scholar]
- 23.Turhan M., Bostanci A., Akdag M. The impact of modified tongue base suspension on CPAP levels in patients with severe OSA. Eur Arch Otorhinolaryngol. 2015;272:995–1000. doi: 10.1007/s00405-014-3034-2. [DOI] [PubMed] [Google Scholar]
- 24.Azbay S., Bostanci A., Aysun Y., Turhan M. The influence of multilevel upper airway surgery on CPAP tolerance in non-responders to obstructive sleep apnea surgery. Eur Arch Otorhinolaryngol. 2016;273:2813–2818. doi: 10.1007/s00405-015-3865-5. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzi-Filho G., Almeida F.R., Strollo P.J. Treating OSA: current and emerging therapies beyond CPAP. Respirology. 2017;22:1500–1507. doi: 10.1111/resp.13144. [DOI] [PubMed] [Google Scholar]
- 26.Fujita S., Conway W., Zorick F., Roth T. Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg. 1981;89:923–934. doi: 10.1177/019459988108900609. [DOI] [PubMed] [Google Scholar]
- 27.Powell N., Guilleminault C., Riley R., Smith L. Mandibular advancement and obstructive sleep apnea syndrome. Bull Eur Physiopathol Respir. 1983;19:607–610. [PubMed] [Google Scholar]
- 28.Liu S.Y., Huon L.K., Powell N.B., Riley R., Cho H.G., Torre C. Lateral pharyngeal wall tension after maxillomandibular advancement for obstructive sleep apnea is a marker for surgical success: observations from drug-induced sleep endoscopy. J Oral Maxillofac Surg. 2015;73:1575–1582. doi: 10.1016/j.joms.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Greenburg D.L., Lettieri C.J., Eliasson A.H. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535–542. doi: 10.1016/j.amjmed.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 30.Fritscher L.G., Mottin C.C., Canani S., Chatkin J.M. Obesity and obstructive sleep apnea–hypopnea syndrome: the impact of bariatric surgery. Obes Surg. 2007;17:95–99. doi: 10.1007/s11695-007-9012-7. [DOI] [PubMed] [Google Scholar]
- 31.Li H.Y., Lee L.A., Wang P.C., Chen N.H., Lin Y., Fang T.J. Nasal surgery for snoring in patients with obstructive sleep apnea. Laryngoscope. 2008;118:354–359. doi: 10.1097/MLG.0b013e318158f73f. [DOI] [PubMed] [Google Scholar]
- 32.Li H.Y., Engleman H., Hsu C.Y., Izci B., Vennelle M., Cross M. Acoustic reflection for nasal airway measurement in patients with obstructive sleep apnea/hypopnea syndrome. Sleep. 2005;28:1554–1559. doi: 10.1093/sleep/28.12.1554. [DOI] [PubMed] [Google Scholar]
- 33.Li H.Y., Wang P.C., Hsu C.Y., Lee S.W., Chen N.H., Liu S.A. Concomitant nasal and palatopharyngeal surgery for obstructive sleep apnea: simultaneous or staged. Acta Otolaryngol. 2005;125:298–303. doi: 10.1080/00016480410022831. [DOI] [PubMed] [Google Scholar]
- 34.Li H.Y., Lee L.A., Wang P.C., Fang T.J., Chen N.H. Can nasal surgery improve obstructive sleep apnea: subjective or objective? Am J Rhinol Allergy. 2009;23:e51–e55. doi: 10.2500/ajra.2009.23.3358. [DOI] [PubMed] [Google Scholar]
- 35.Li H.Y., Wang P.C., Chen Y.P., Lee L.A., Fang T.J., Lin H.C. Critical appraisal and meta-analysis of nasal surgery for obstructive sleep apnea. Am J Rhinol Allergy. 2011;25:45–49. doi: 10.2500/ajra.2011.25.3558. [DOI] [PubMed] [Google Scholar]
- 36.Friedman M., Tanyeri H., Lim J.W., Landsberg R., Vaidyanathan K., Caldarelli D. Effect of improved nasal breathing on obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;122:71–74. doi: 10.1016/S0194-5998(00)70147-1. [DOI] [PubMed] [Google Scholar]
- 37.Cahali M.B. Lateral pharyngoplasty: a new treatment for obstructive sleep apnea hypopnea syndrome. Laryngoscope. 2003;113:1961–1968. doi: 10.1097/00005537-200311000-00020. [DOI] [PubMed] [Google Scholar]
- 38.Li H.Y., Lee L.A. Relocation pharyngoplasty for obstructive sleep apnea. Laryngoscope. 2009;119:2472–2477. doi: 10.1002/lary.20634. [DOI] [PubMed] [Google Scholar]
- 39.Li H.Y., Lee L.A., Fang T.J., Lin W.Y. Evaluation of velopharyngeal function after relocation pharyngoplasty for obstructive sleep apnea. Laryngoscope. 2010;120:1069–1073. doi: 10.1002/lary.20850. [DOI] [PubMed] [Google Scholar]
- 40.Li H.Y. Updated palate surgery for obstructive sleep apnea. Adv Otorhinolaryngol. 2017;80:74–80. doi: 10.1159/000470869. [DOI] [PubMed] [Google Scholar]
- 41.Vicini C., Hendawy E., Campanini A., Eesa M., Bahgat A., AlGhamdi S. Barbed reposition pharyngoplasty (BRP) for OSAHS: a feasibility, safety, efficacy and teachability pilot study. ‘‘We are on the giant's shoulders”. Eur Arch Otorhinolaryngol. 2015;272:3065–3070. doi: 10.1007/s00405-015-3628-3. [DOI] [PubMed] [Google Scholar]
- 42.Vicini C., Dallan I., Canzi P., Frassineti S., Nacci A., Seccia V. Transoral robotic surgery of the tongue base in obstructive sleep Apnea-Hypopnea syndrome: anatomic considerations and clinical experience. Head Neck. 2012;34:15–22. doi: 10.1002/hed.21691. [DOI] [PubMed] [Google Scholar]
- 43.Li H.Y., Lee L.A., Kezirian E.J. Coblation endoscopic lingual lightening (CELL) for obstructive sleep apnea. Eur Arch Otorhinolaryngol. 2016;273:231–236. doi: 10.1007/s00405-014-3475-7. [DOI] [PubMed] [Google Scholar]
- 44.Li H.Y., Lee L.A., Kezirian E.J. Efficacy of coblation endoscopic lingual lightening in multilevel surgery for obstructive sleep apnea. JAMA Otolaryngol Head Neck Surg. 2016;142:438–443. doi: 10.1001/jamaoto.2015.3859. [DOI] [PubMed] [Google Scholar]
- 45.Kezirian E.J., Goldberg A.N. Hypopharyngeal surgery in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2006;132:206–213. doi: 10.1001/archotol.132.2.206. [DOI] [PubMed] [Google Scholar]
- 46.Li H.Y., Lo Y.L., Wang C.J., Hsin L.J., Lin W.N., Fang T.J. Dynamic drug- induced sleep computed tomography in adults with obstructive sleep apnea. Sci Rep. 2016;6:35849. doi: 10.1038/srep35849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kezirian E.J., Hohenhorst W., de Vries N. Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol. 2011;268:1233–1236. doi: 10.1007/s00405-011-1633-8. [DOI] [PubMed] [Google Scholar]
- 48.Chung J.W., Enciso R., Levendowski D.J., Westbrook P.R., Clark G.T. Patients with positional versus nonpositional obstructive sleep apnea: a retrospective study of risk factors associated with apnea-hypopnea severity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:605–610. doi: 10.1016/j.tripleo.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 49.Villa M.P., Brasili L., Ferretti A., Vitelli O., Rabasco J., Mazzotta A.R. Oropharyngeal exercises to reduce symptoms of OSA after AT. Sleep Breath. 2015;19:281–289. doi: 10.1007/s11325-014-1011-z. [DOI] [PubMed] [Google Scholar]
- 50.Villa M.P., Evangelisti M., Martella S., Barreto M., Del Pozzo M. Can myofunctional therapy increase tongue tone and reduce symptoms in children with sleep-disordered breathing? Sleep Breath. 2017;21:1025–1032. doi: 10.1007/s11325-017-1489-2. [DOI] [PubMed] [Google Scholar]
- 51.Guilleminault C., Huang Y.S., Monteyrol P.J., Sato R., Quo S., Lin C.H. Critical role of myofascial reeducation in pediatric sleep-disordered breathing. Sleep Med. 2013;14:518–525. doi: 10.1016/j.sleep.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 52.Camacho M., Guilleminault C., Wei J.M., Song S.A., Noller M.W., Reckley L.K. Oropharyngeal and tongue exercises (myofunctional therapy) for snoring: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2018;275:849–855. doi: 10.1007/s00405-017-4848-5. [DOI] [PubMed] [Google Scholar]
- 53.Guimarães K.C., Drager L.F., Genta P.R., Marcondes B.F., Lorenzi-Filho G. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2009;179:962–966. doi: 10.1164/rccm.200806-981OC. [DOI] [PubMed] [Google Scholar]
- 54.Camacho M., Certal V., Abdullatif J., Zaghi S., Ruoff C.M., Capasso R. Myofunctional therapy to treat obstructive sleep apnea: a systematic review and meta-analysis. Sleep. 2015;38:669–675. doi: 10.5665/sleep.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee S.Y., Guilleminault C., Chiu H.Y., Sullivan S.S. Mouth breathing,“nasal disuse,” and pediatric sleep-disordered breathing. Sleep Breath. 2015;19:1257–1264. doi: 10.1007/s11325-015-1154-6. [DOI] [PubMed] [Google Scholar]
- 56.Oksenberg A., Silverberg D.S., Arons E., Radwan H. Positional vs nonpositional obstructive sleep apnea patients: anthropomorphic, nocturnal polysomnographic and multiple sleep latency test data. Chest. 1997;112:629–639. doi: 10.1378/chest.112.3.629. [DOI] [PubMed] [Google Scholar]
- 57.de Vries G.E., Hoekema A., Doff M.H., Kerstjens H.A., Meijer P.M., van der Hoeven J.H. Usage of positional therapy in adults with obstructive sleep apnea. J Clin Sleep Med. 2015;11:131–137. doi: 10.5664/jcsm.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ravesloot M.J., White D., Heinzer R., Oksenberg A., Pépin J.L. Efficacy of the new generation of devices for positional therapy for patients with positional obstructive sleep apnea: a systematic review of the literature and meta-analysis. J Clin Sleep Med. 2017;13:813–824. doi: 10.5664/jcsm.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee C.H., Kim S.W., Han K., Shin J.M., Hong S.L., Lee J.E. Effect of uvulopalatopharyngoplasty on positional dependency in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2011;137:675–679. doi: 10.1001/archoto.2011.99. [DOI] [PubMed] [Google Scholar]
- 60.Carter R., 3rd, Watenpaugh D.E. Obesity and obstructive sleep apnea: or is it OSA and obesity? Pathophysiology. 2008;15:71–77. doi: 10.1016/j.pathophys.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 61.Dobrosielski D.A., Papandreou C., Patil S.P., Salas-Salvadó J. Diet and exercise in the management of obstructive sleep apnoea and cardiovascular disease risk. Eur Respir Rev. 2017;26:160110. doi: 10.1183/16000617.0110-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johansson K., Neovius M., Lagerros Y.T., Harlid R., Rössner S., Granath F. Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese men: a randomised controlled trial. BMJ. 2009;339:b4609. doi: 10.1136/bmj.b4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith S., Sullivan K., Hopkins W., Douglas J. Frequency of insomnia report in patients with obstructive sleep apnoea hypopnea syndrome (OSAHS) Sleep Med. 2004;5:449–456. doi: 10.1016/j.sleep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 64.Krakow B., Melendrez D., Ferreira E., Clark J., Warner T.D., Sisley B. Prevalence of insomnia symptoms in patients with sleep-disordered breathing. Chest. 2001;120:1923–1929. doi: 10.1378/chest.120.6.1923. [DOI] [PubMed] [Google Scholar]
- 65.Benetó A., Gomez-Siurana E., Rubio-Sanchez P. Comorbidity between sleep apnea and insomnia. Sleep Med Rev. 2009;13:287–293. doi: 10.1016/j.smrv.2008.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.