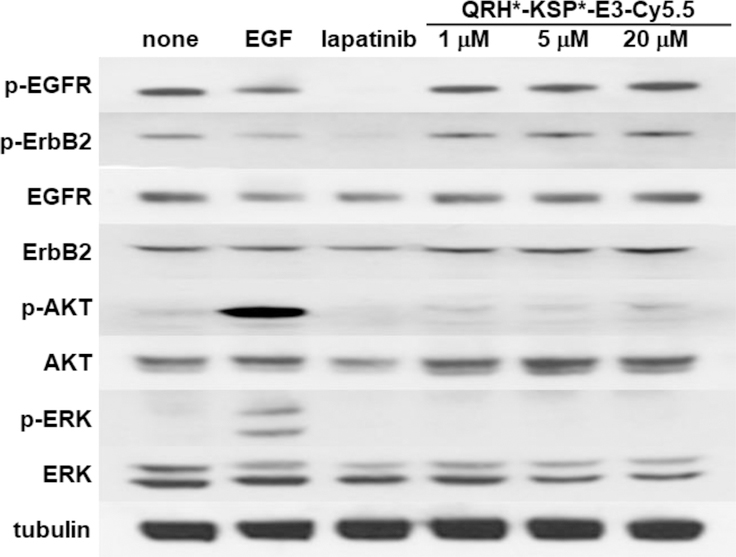
Figure 4. No effect of peptide heterodimer on cell signaling.
We evaluated the effect of the QRH*-KSP*-E3-Cy5.5 on downstream cell signaling after binding to SKBr3 cells. On Western blot, we observed no change in phosphorylation of EGFR (p-EGFR), ErbB2 (p-ErbB2) or of downstream AKT (p-AKT) and ERK (p-ERK) with incubation of heterodimer at 1, 5, and 20 μM. By comparison, the addition of EGF, an endogenous ligand for EGFR, showed increased expression of p-AKT and p-ERK. The addition of 100 nM of lapatinib, a tyrosine kinase inhibitor known to interrupt EGFR/ErbB2 signaling in solid tumors, showed reduced expression of p-EGFR, p-ErbB2 and p-AKT. Cells treated with 1% DMSO and untreated cells showed no suppression of EGFR and ErbB2 mediated signaling. β-tubulin is used as loading control.