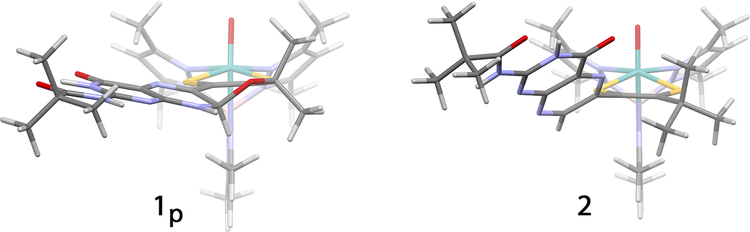
Figure 3.
(Left) X-ray structure of 1p shows that pyran formation enforces a nearly coplanar arrangement of the dithiolene and pterin systems, with the angle between the dithiolene chelate and the pterin rings 40° out of planarity being τ = 9°. (Right) The DFT optimized structure of 2 shows the pterin rotated τ ≈ with the dithiolene due to a steric repulsion between the t-butyl group and the pterin.