Abstract
C-type lectins of the Reg3 family belong to antimicrobial peptides (AMPs), which function as a barrier to protect body surfaces against microorganisms. Reg3 mainly expressed throughout the small intestine modulate host defense process via bactericidal activity. A wide range of studies indicate that Reg3 family plays an important role in the physical segregation of microbiota from host as well as the immune response induced by enteric pathogens. In this review, we review a growing literature on the potential metabolic functions of Reg3 proteins and their potential to act as important gut hormones.
The gastrointestinal (GI) tract is exposed to extrinsic diets and pathogens. The result is that inside the lumen, the host interacts with a variety of bacteria, which requires multiple immune responses and results in production of metabolites that contribute to the intestinal microenvironment (1–5). Because the gut epithelium is directly facing these gut bacteria (termed the microbiota), the host must rely on a diverse set of physical and biochemical barriers to keep these pathogens from getting into the body. These include immunoglobulin A, the mucus layer, tight junctions, and antimicrobial peptides (AMPs) (6). AMPs have been found from prokaryote to human as a part of the innate immune response (7, 8). AMPs have essential roles in protecting mammalian body surfaces, which directly interface with the external microorganisms (9). Multiple antimicrobial peptides, including defensins, lysozymes, and C-type lectins, are produced by epithelial cells of tissues including skin, respiratory tract, and intestine as well as circulating immune cells (10–13), and contribute to the host defense against microorganisms via a variety of innate immune responses (14, 15). Among those AMPs, Reg3 lectins are abundantly expressed in enterocyte and paneth cells of small intestine and exert bactericidal activity as a host defense mechanism in the gut (12, 16). In this review, we focus on the emerging role of Reg3 proteins beyond this traditional role to regulate systemic metabolism.
Discovery of the Reg3
Reg proteins, which are part of the C-type lectins, were first discovered in regenerating islets (17). Various Reg family members have been independently found classified into four subtypes (types I, II, III, and IV) based on their DNA sequence and primary structures of the encoded sequence homology (18–22). During the same time frame, other investigators identified these same proteins that were overexpressed in rat acute pancreatitis or human hepatocellular carcinoma and termed them pancreatitis-associated protein/hepatic intestinal pancreatic protein (21, 23, 24). Reg3 proteins can be divided into multiple subtypes (α, β, γ, and δ) determined by their structure and chromosomal localization (22, 25). It is known that there are four Reg3 members termed Reg3α, Reg3β, Reg3δ, and Reg3γ in mice, whereas humans are known to have only Reg3α and Reg3γ (21, 26). In addition, islet neogenesis-associated protein, Reg3δ in hamsters, was discovered in a surgical model of partial duct obstruction in hamster pancreas (27–29).
Cellular Source and Secretion of Reg3
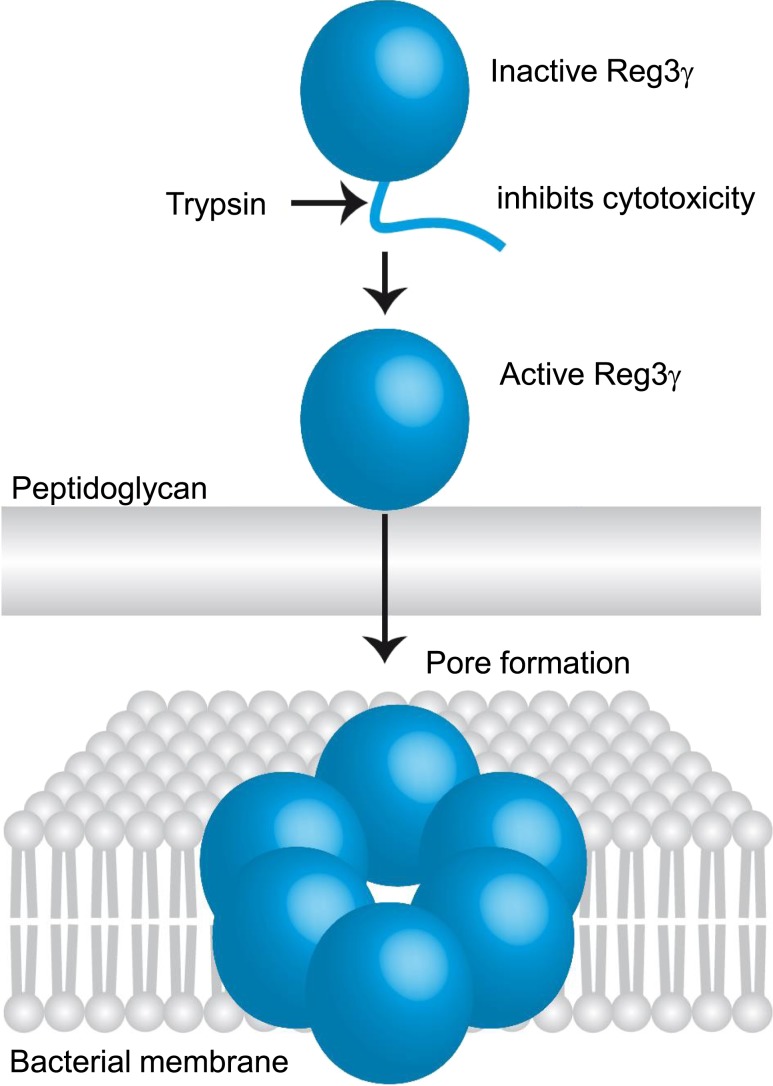
The Reg3 gene is divided into 6 exons and encodes a protein that is 175 amino acids in length with a molecular mass of 16 to 17 kDa (25, 30, 31). As a member of the Reg3 subgroup, Reg3γ (the mouse homolog of human REG3A) contains an anionic N-terminal prosegment that maintains the protein in a biologically inactive state. The inactive prosegment is removed by trypsin-dependent proteolytic processing, which leads inactive Reg3γ to become active Reg3γ with bactericidal activity (Fig. 1) (31, 32). According to RNA and protein data from the Human Protein Atlas, REG3Α is expressed at the highest levels in the GI tract (www.proteinatlas.org). Its expression is triggered by bacterial colonization (12, 33) and upregulated in inflammatory bowel disease (16).
Figure 1.
Mechanism of Reg3γ activity. Active Reg3γ is processed by trypsin-dependent proteolysis. Reg3γ targets Gram-positive bacteria by binding to peptidoglycan layer. Then, Reg3γ exert bactericidal activity by oligomerizing to form hexameric transmembrane pores. [Reprinted with permission from Springer Nature. Nature; Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Sohini Jukherjee, Jui Zheng, Mehabaw G. Derebe, Keith M. Callenberg, Carrie L. Partch, et al., Nov. 20, 2013.]
Whereas Reg3γ expression is more restricted in the intestine under normal conditions, it has been known for over a decade that the expression of intestinal Reg3 is influenced by various factors (Table 1). For example, microbial stimulation is required for the expression of the Reg3 protein because its expression is suppressed by inhibiting bacterial colonization under germ-free conditions or antibiotic treatment (12, 34), whereas supplementation with specific bacteria enhances Reg3 expression (35, 36). Nutritional (high-fat diet, prebiotics, and alcohol) (37–41) or surgery (graft-versus-host disease by an allogeneic transplantation or bariatric surgery) (42–45) can also alter Reg3γ expression (Table 1). Although bariatric surgery resulted in increased levels of intestinal Reg3γ and Reg3β (42, 43), it is still unclear whether this change is an effect of bariatric surgery or a cause of metabolic improvements of surgery. Additionally, studies show that bile acids and GLP-1 play a role in the regulation of the intestinal and pancreatic Reg3 expression, respectively (46, 47). Interestingly, recent findings implicate its expression in other tissues under disease status. For instance, Reg3γ is upregulated in epidermal keratinocytes with skin lesions, such as aseptic wounds or psoriasis (48, 49), as well as in pancreas and colon during pancreatitis and inflammatory bowel disease (16, 50). However, the precise cellular source of Reg3 proteins in disease status still needs to be elucidated.
Table 1.
Factors That Affect Reg3γ Expression
| Factors | Gene Expression | Tissue/Location |
|---|---|---|
| Germ-free | Decrease | Small intestine |
| HFD | Decrease | Small intestine, colon |
| Antibiotics | Decrease | Small intestine |
| Alcohol | Decrease | Small intestine |
| Pre-(probiotics) | Increase | Small intestine, colon |
| Bariatric surgery | Increase | Small intestine |
| GVHD | Decrease | Small intestine |
| Increase | Blood | |
| Exendin-4 treatment | Increase | Pancreas |
| Bile acid | Increase | Small intestine |
| Skin wound | Increase | Skin |
| Pancreatitis | Increase | Pancreas |
| Inflammatory bowel disease | Increase | Colon |
Abbreviations: GVHD, graft-versus-host disease; HFD, high-fat diet.
Reg3 measurement is rarely carried out by ELISAs in plasma or serum. According to previous studies, human REG3A (human homolog of mouse Reg3γ) and mouse Reg3γ concentration of healthy subjects is almost stable to 15 to 25 ng/mL (44, 45, 51). Although the expression of Reg3 in tissues has been measured by quantitative real-time PCR or western blotting in most of the rodent studies, there seem to be no appropriate antibodies for ELISA kits available in the market to quantify circulating Reg3γ levels. We used a variety of ELISAs to assess circulating levels, including the one previously used by Zhao et al. (44). However, all of these assays detected substantial levels of Reg3γ in Reg3γ knockout mice, indicating that there is cross-reactivity with other circulating proteins.
Putative Receptor for Reg3 Signaling
Kobayashi et al. (52) isolated Reg-binding protein that is encoded by the exostose-like glycosyltransferase (EXTL) gene in the screening of the rat islet cDNA library. EXTL3 has been implicated as a potential receptor for Reg3 because Extl3 has been shown to bind to Reg3γ protein in keratinocyte (48) (Table 2). EXTL3 is expressed in various tissues such as skin, liver, kidney, adipose tissue, stomach, small intestine, colon, pancreas, adrenal gland, pituitary gland, and brain (www.proteinatlas.org) (48, 52). In addition, EXTL3 is abundant in hematopoietic stem cells and early T cells (53). Most of what is known about EXTL3 is from studies of heparan sulfate/heparin biosynthesis (54, 55). Genetic loss of function of Extl3 is lethal to the embryo due to a defect in heparan sulfate synthesis (55). Evidence for Extl3 as a binding protein for Reg1 was first demonstrated in pancreatic β-cells (52). At the same time, Mueller et al. (56) suggested that Reg1 promoted cell growth in rat pancreatic-derived cells by activating the MAPK–c-Jun N-terminal kinase–cyclin D1 pathway via Extl3. Using a human pancreatic cell line, EXTL3 was identified as a potential receptor for Reg3 protein, which promotes its translocation from membrane to nucleus (57). Examining the contribution of Extl3 signaling in islets in β-cell–specific Extl3-deficient mice displayed abnormal islet morphology and glucose intolerance due to impaired insulin secretion (55). A recent study indicates that the Reg3γ-Extl3 binding regulates keratinocyte proliferation and differentiation by activating the phosphatidylinositol 3-kinase (PI3K)-AKT signaling pathway (48). Further, this Reg3γ-Extl3 signaling serves as a mediator for induction of SHP-1 via AKT–signal transducer and activator of transcription (STAT) 3 activation to inhibit Toll-like receptor (TLR) 3–induced inflammation and may be involved in regulating impaired wound healing in diabetes (49). Interestingly, Reg3γ appears to signal through the epidermal growth factor receptor/JAK2/STAT3 pathway in tumors (58). Although there appears to be some overlap in these signaling pathways, the possibility that there might be other receptors for Reg3 proteins needs to be considered.
Table 2.
Reg Proteins Binding to Extl3
| Reg Protein | Signaling Pathway | Function |
|---|---|---|
| Reg1 | Activation of MAPK-JNK-cyclin D1 | Pancreatic cell growth |
| Reg3γ | Activation of PI3K-AKT | Keratinocyte proliferation and differentiation |
| Inhibition of TLR3 via AKT-STAT3-SHP1 | Promoting wound healing |
Abbreviation: JNK, c-Jun N-terminal kinase.
Tissue-Specific Metabolic Actions of the Reg3 Family
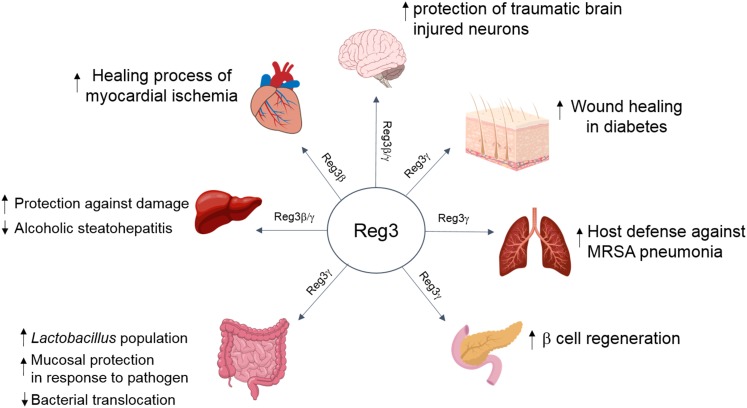
A role for Reg3 has been reported in a wide range of organs, including liver, intestine, pancreas, lung, heart, skin, and nervous system (48, 49, 59–66) (Fig. 2). Later, we review the existing literature that points to a role of the Reg3 family in various aspects of metabolism.
Figure 2.
Physiological effects of Reg3 protein on various organs. Reg3γ protein acts on various organs as well as mediates the multiple benefits, including tissue protection against damage and improvement of the regenerating process. MRSA, methicillin-resistant Staphylococcus aureus.
Pancreas (pancreatic cells)
During organogenesis, the expression of Reg3 proteins are restricted to glucagon-expressing enteropancreatic endocrine cells at 10 weeks of gestation (67). Reg3 was not detected in normal adult islets but in regenerating islets (19). This suggests that Reg3 has important roles in islets responding to pancreatic damage. For example, pancreata lacking Reg3γ showed a reduction in activation of STAT3/SOCS3 signaling, which led to elevated apoptotic and inflammatory responses in acute pancreatitis (68). Xia et al. (59) recently provided evidence for Reg3γ in regulating the JAK2/STAT3 pathway and conferring a beneficial effect on β-cell regeneration. In line with this, Reg3β has a protective effect on streptozotocin-induced pancreatic β-cell damage (69). Intriguingly, Reg3 expression was elevated in pancreas from a model of iron overload (70, 71). Upregulation of the Reg3 family is likely attributable to pancreatic damage to reduce iron-induced pancreatic stress because it is known that excessive iron can result in iron deposition in islets, β-cell dysfunction, and impaired insulin secretion (72). Such findings point toward the possibility that Reg3γ signaling confers beneficial effects on damaged cells. Conversely, it is important to note that the influence of Reg3γ is likely to be dependent on the changes in microenvironment. For example, Reg3γ may affect tumor suppressor function of CD8+ T cells and act as oncogenic signaling in pancreatic cancer via activating the epidermal growth factor receptor/JAK2/STAT3 signaling pathway (58, 73), which reflect that the effect of Reg3 may be different depending on immune status.
Liver
In normal adult and fetal liver, Reg3 mRNA expression is not detected, whereas it is highly expressed in hepatocarcinoma (23). Studies using mouse models have demonstrated that intestinal Reg3β/γ plays a role in protecting alcohol-induced liver disease by inhibiting bacterial translocation (39, 60). Furthermore, ectopic expression of Reg3β in hepatocytes has been found to act on multiple aspects of liver regeneration in a variety of liver damage models (74–76). Reg3 in hepatocytes stimulates antiapoptotic activity via increased protein kinase A (PKA), which leads to liver regeneration in partial hepatectomy (76). Furthermore, Reg3β protects from drug-induced liver injury by inhibiting oxidative damage as well as elevating antioxidant (superoxide dismutase and glutathione reductase activity) activity (74, 75). Taken together, these data suggest that effects of Reg3 proteins are minimal under normal healthy conditions but may be of importance to liver, pancreas, and intestine under pathological conditions.
Skin
A few studies have reported the effects of Reg3γ on epidermal keratinocytes around skin wounds. Reg3γ is highly expressed in patients with psoriasis and mice with either drug-induced psoriatic skin lesions or physical skin wound (48). The elevation of Reg3γ induced by IL-17 enhances keratinocyte proliferation and inhibits differentiation through the EXTL3-PI3K-AKT signaling pathway. In contrast to skin wounds in normal patients, wound-healing processes in patients with diabetes are disrupted due to both hyperglycemia and dysfunctional immune response (77, 78). Compared with patients without diabetes, Reg3γ expression around skin wounds of patients with diabetes was suppressed (49).
Adipose tissue
There are studies illustrating a role of the Reg3 family in adipose tissue. A recent study showed that either deficiency or overexpression of Reg3γ or Reg3β has no effect on diet-induced obesity and glucose tolerance. However, Secq et al. (79) reported Reg3γ as an obesogenic factor with the observation that mice expressing human REG3A specifically in the liver became obese on a normal chow diet as well as circulating REG3A levels were elevated in obese patients. At the same time, examining the contribution of REG3A through 3T3-L1 cells showed increased cell growth mediated by the MAPK kinase/ERK signaling pathway. A caveat of this study is that the analysis is performed in undifferentiated preadipocytes; therefore, the role of Reg3 family in adipocyte differentiation or maturation remains unclear. At the same time, these authors took advantage of transgenic mice overexpressing human REG3Α specifically in the hepatocytes. Therefore, a mechanism to explain the cell-specific actions of Reg3 needs to be determined. Likewise, although transcriptional levels of Reg3 were remarkably upregulated in mesenteric fat in response to a high-fat diet (80), it cannot be ruled out that mesenteric fat tissue would be contaminated with mesenteric lymph node, which contains numerous immune cells. Intriguingly, another recent study showed that mice overexpressing human REG3γ in gut epithelial cells were protected from diet-induced obesity and improved glucose metabolism (81). Further, Reg3γ transgenic overexpressing mice showed that Reg3γ-associated Lactobacillus influence anti-inflammatory macrophage pools not only in the gut but also in the spleen and adipose tissues (82). This would implicate Reg3 signaling in the regulation of body weight and glucose metabolism via actions on the microbiota and immune system. However, the discrepancy in phenotypes in adipose tissue requires further efforts to find the direct contribution of Reg3 protein in adipose tissue in obesity development. Although there is a possibility that actions of Reg3 proteins are mediated via a specific receptor for Reg3 proteins in adipose tissue, the mechanism underlying the adipose tissue by Reg3 proteins remains unclear.
Intestine
Reg3γ has a potent bactericidal effect on Gram-positive bacteria by binding to a surface peptidoglycan layer, followed by forming a membrane-penetrating pore (32). Reg3β, a Reg3 murine isoform, which is abundantly expressed in the GI tract, binds directly to lipopolysaccharide and exerts bactericidal activity against Gram-negative bacteria (83). These Reg3 proteins are crucial for maintaining spatial segregation between host and gut bacteria (64). In line with this action against bacteria, Reg3γ restricts mucosa-associated bacterial colonization and decreases bacterial translocation in alcohol-fed mice, which resulted in resistance to alcohol-induced liver damage (60). Interestingly, either gain or loss of Reg3γ did not influence metabolic features in diet-induced nonalcoholic steatohepatitis (82). This result may be due to the insufficient induction of bacterial translocation and endotoxemia when challenged with a western-style diet. Another function of Reg3 in the gut is to mediate host barrier function as a downstream target of IL-22 (84). Microorganism-associated molecular patterns also influence the expression of Reg3γ in the intestine through TLRs (10). For instance, systemic administration of TLR ligand flagellin-induced Reg3γ expression in the gut epithelial cells by TLR5 stimulation and IL-22 secretion, which protects mice from antibiotic-resistant bacterial colonization (85). Further, oral administration of lipopolysaccharide triggered Reg3γ expression through TLR4 stimulation, thereby enhancing immune resistance to infection with antibiotic-resistant bacteria (34). Recent studies reported that both Reg3β and Reg3γ also influence epithelial proliferation, crypt regeneration, and protection of intestinal stem cells and Paneth cells from apoptosis in the tissue damage (44, 86, 87).
Future Directions
Taken together, these studies indicate that Reg3 proteins activity may be beneficial in metabolic regulation but many questions remain unanswered. For example, it is uncertain whether Reg3 proteins produced in the intestine are delivered to target tissues as a circulating hormone or whether local production of Reg3 proteins are produced in certain cells such as macrophages, innate lymphoid cells that may act in a paracrine manner in various tissues. On a related note of signaling transduction, it needs to be determined whether Reg3γ exerts its metabolic effects by binding to a specific receptor such as EXTL3 that is found in many tissues including brain, liver, fat, and pancreas. Although Lai et al. (48) suggested that the Reg3γ-EXTL3 complex promotes skin wound healing through PI3K-AKT activation, more research is required to understand tissue-specific Reg3 signaling pathway. Furthermore, because Extl3 has been detected throughout the brain and it also contributes to brain development and corticogenesis (88–90), potential effects of Reg3γ in the central nervous system on food intake or glucose homeostasis need to be investigated.
In addition, future studies need to focus on effects of Reg3 molecules on shaping the microbiota composition. Although it has been known that Reg3γ exerts bactericidal activity against Gram-positive bacteria (32), substantial evidence that increases in portions of Lactobacillus belonging to Gram-positive bacteria is affected by Reg3γ (81) suggests that there may be certain population of microbiota that is resistant to Reg3γ or stimulated by Reg3γ.
Finally, it remains to be investigated whether Reg3 proteins are necessary for the beneficial effects of bariatric surgery. Bariatric surgery is currently acknowledged as the most effective treatment of obesity and glucose regulation (91, 92). Roux-en-Y gastric bypass and vertical sleeve gastrectomy are the most common procedures. Besides the weight loss, anatomical alteration of the GI tract by surgery influences gut hormone secretion, composition of bile acid and microbiota, and changes in micronutrients including iron deficiency (93–99). These studies indicate that surgery likely alters the gut microenvironment, such as intestinal barrier function, immune system, and enteroendocrine activity. Intriguingly, studies exploring molecular response in gastric bypass surgery showed that Reg3γ and Reg3β were upregulated in the jejunum after surgery (42, 43). Thus, it is worth considering the impact of Reg3 proteins on the sustained body weight loss and improved glucose regulation after bariatric surgery. Such work could directly implicate Reg3 proteins as gut hormones for which levels can be manipulated for potential therapeutic benefit.
Summary
Based on the existing evidence described in this review, we suggest the importance of Reg3 protein for metabolic homeostasis in multiple organs. Studies demonstrated that Reg3 protein protects β-cell, hepatocyte, and keratinocyte from damages and promotes cell regeneration (48, 49, 59, 69, 74–76). Reg3γ is primarily produced in the GI tract, and in general, Reg3 proteins play a role in protection against damage, cell proliferation, antibacterial defense, and inhibition of bacterial translocation (64, 100). Together, it remains an open question as to whether the endocrine or paracrine actions of Reg3 protein regulate key metabolic processes. Nevertheless, accumulating evidence that tissue specifically overexpressed the Reg3 gene resulted in better systemic metabolism via microbiota alteration or the administration of extrinsic Reg3 protein improved β-cell function has indicated the potential of Reg3 proteins as endocrine action or autocrine/paracrine action (69, 81, 101). Although we are still awaiting to determine the circulating action vs local paracrine or autocrine action of Reg3 proteins, such work could implicate Reg3 proteins as gut hormones for which levels can be manipulated for potential therapeutic benefit.
Acknowledgments
Financial Support: R.J.S. is supported by National Institutes of Health Grant R01DK107652. J.H.S. is supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2018R1A6A3A03011269).
Disclosure Summary: R.J.S. has received research support from Ethicon Endo-Surgery, Novo Nordisk, Janssen, Zafgen, Kallyope, and MedImmune; served on scientific advisory boards for Ethicon Endo-Surgery, Novo Nordisk, Janssen, Kallyope, Scophia, Sanofi, and Ironwood Pharmaceuticals; and served as a paid consultant for Novo Nordisk and Zafgen. The remaining author has nothing to disclose.
Glossary
Abbreviations:
- AMP
antimicrobial peptide
- EXTL
exostose-like glycosyltransferase
- GI
gastrointestinal
- PI3K
phosphatidylinositol 3-kinase
- PKA
protein kinase A
- STAT
signal transducer and activator of transcription
- TLR
Toll-like receptor
References and Notes
- 1. Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13(5):517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sun M, He C, Cong Y, Liu Z. Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol. 2015;8(5):969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Byndloss MX, Olsan EE, Rivera-Chávez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y, Litvak Y, Lopez CA, Xu G, Napoli E, Giulivi C, Tsolis RM, Revzin A, Lebrilla CB, Bäumler AJ. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357(6351):570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schirbel A, Kessler S, Rieder F, West G, Rebert N, Asosingh K, McDonald C, Fiocchi C. Pro-angiogenic activity of TLRs and NLRs: a novel link between gut microbiota and intestinal angiogenesis. Gastroenterology. 2013;144(3):613–623.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cani PD. Interactions between gut microbes and host cells control gut barrier and metabolism. Int J Obes Suppl. 2016;6(Suppl 1)S28–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kelsen JR, Baldassano RN, Artis D, Sonnenberg GF. Maintaining intestinal health: the genetics and immunology of very early onset inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2015;1(5):462–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beisswenger C, Bals R. Functions of antimicrobial peptides in host defense and immunity. Curr Protein Pept Sci. 2005;6(3):255–264. [DOI] [PubMed] [Google Scholar]
- 8. Mahlapuu M, Håkansson J, Ringstad L, Björn C. Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol. 2016;6:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395. [DOI] [PubMed] [Google Scholar]
- 10. Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12(7):503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Müller CA, Autenrieth IB, Peschel A. Innate defenses of the intestinal epithelial barrier. Cell Mol Life Sci. 2005;62(12):1297–1307. [DOI] [PubMed] [Google Scholar]
- 12. Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313(5790):1126–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjöberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, Stoel M, Zhou Y, Sodergren E, Weinstock GM, Bevins CL, Williams CB, Bos NA. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11(1):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30(3):131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mukherjee S, Vaishnava S, Hooper LV. Multi-layered regulation of intestinal antimicrobial defense. Cell Mol Life Sci. 2008;65(19):3019–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ogawa H, Fukushima K, Naito H, Funayama Y, Unno M, Takahashi K, Kitayama T, Matsuno S, Ohtani H, Takasawa S, Okamoto H, Sasaki I. Increased expression of HIP/PAP and regenerating gene III in human inflammatory bowel disease and a murine bacterial reconstitution model. Inflamm Bowel Dis. 2003;9(3):162–170. [DOI] [PubMed] [Google Scholar]
- 17. Terazono K, Yamamoto H, Takasawa S, Shiga K, Yonemura Y, Tochino Y, Okamoto H. A novel gene activated in regenerating islets. J Biol Chem. 1988;263(5):2111–2114. [PubMed] [Google Scholar]
- 18. Hartupee JC, Zhang H, Bonaldo MF, Soares MB, Dieckgraefe BK. Isolation and characterization of a cDNA encoding a novel member of the human regenerating protein family: Reg IV. Biochim Biophys Acta. 2001;1518(3):287–293. [DOI] [PubMed] [Google Scholar]
- 19. Suzuki Y, Yonekura H, Watanabe T, Unno M, Moriizumi S, Miyashita H, Okamoto H. Structure and expression of a novel rat RegIII gene. Gene. 1994;144(2):315–316. [DOI] [PubMed] [Google Scholar]
- 20. Unno M, Yonekura H, Nakagawara K, Watanabe T, Miyashita H, Moriizumi S, Okamoto H, Itoh T, Teraoka H. Structure, chromosomal localization, and expression of mouse reg genes, reg I and reg II. A novel type of reg gene, reg II, exists in the mouse genome. J Biol Chem. 1993;268(21):15974–15982. [PubMed] [Google Scholar]
- 21. Iovanna J, Orelle B, Keim V, Dagorn JC. Messenger RNA sequence and expression of rat pancreatitis-associated protein, a lectin-related protein overexpressed during acute experimental pancreatitis. J Biol Chem. 1991;266(36):24664–24669. [PubMed] [Google Scholar]
- 22. Abe M, Nata K, Akiyama T, Shervani NJ, Kobayashi S, Tomioka-Kumagai T, Ito S, Takasawa S, Okamoto H. Identification of a novel Reg family gene, Reg IIIdelta, and mapping of all three types of Reg family gene in a 75 kilobase mouse genomic region. Gene. 2000;246(1-2):111–122. [DOI] [PubMed] [Google Scholar]
- 23. Lasserre C, Christa L, Simon MT, Vernier P, Bréchot C. A novel gene (HIP) activated in human primary liver cancer. Cancer Res. 1992;52(18):5089–5095. [PubMed] [Google Scholar]
- 24. Keim V, Rohr G, Stöckert HG, Haberich FJ. An additional secretory protein in the rat pancreas. Digestion. 1984;29(4):242–249. [DOI] [PubMed] [Google Scholar]
- 25. Narushima Y, Unno M, Nakagawara K, Mori M, Miyashita H, Suzuki Y, Noguchi N, Takasawa S, Kumagai T, Yonekura H, Okamoto H. Structure, chromosomal localization and expression of mouse genes encoding type III Reg, RegIII α, RegIII β, RegIII γ. Gene. 1997;185(2):159–168. [DOI] [PubMed] [Google Scholar]
- 26. Okamoto H. The Reg gene family and Reg proteins: with special attention to the regeneration of pancreatic beta-cells. J Hepatobiliary Pancreat Surg. 1999;6(3):254–262. [DOI] [PubMed] [Google Scholar]
- 27. Lipsett M, Hanley S, Castellarin M, Austin E, Suarez-Pinzon WL, Rabinovitch A, Rosenberg L. The role of islet neogenesis-associated protein (INGAP) in islet neogenesis. Cell Biochem Biophys. 2007;48(2-3):127–137. [DOI] [PubMed] [Google Scholar]
- 28. Rosenberg L, Brown RA, Duguid WP. A new approach to the induction of duct epithelial hyperplasia and nesidioblastosis by cellophane wrapping of the hamster pancreas. J Surg Res. 1983;35(1):63–72. [DOI] [PubMed] [Google Scholar]
- 29. Parikh A, Stephan A-F, Tzanakakis ES. Regenerating proteins and their expression, regulation and signaling. Biomol Concepts. 2012;3(1):57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nata K, Liu Y, Xu L, Ikeda T, Akiyama T, Noguchi N, Kawaguchi S, Yamauchi A, Takahashi I, Shervani NJ, Onogawa T, Takasawa S, Okamoto H. Molecular cloning, expression and chromosomal localization of a novel human REG family gene, REG III. Gene. 2004;340(1):161–170. [DOI] [PubMed] [Google Scholar]
- 31. Mukherjee S, Partch CL, Lehotzky RE, Whitham CV, Chu H, Bevins CL, Gardner KH, Hooper LV. Regulation of C-type lectin antimicrobial activity by a flexible N-terminal prosegment. J Biol Chem. 2009;284(8):4881–4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mukherjee S, Zheng H, Derebe MG, Callenberg KM, Partch CL, Rollins D, Propheter DC, Rizo J, Grabe M, Jiang QX, Hooper LV. Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature. 2014;505(7481):103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Syder AJ, Oh JD, Guruge JL, O’Donnell D, Karlsson M, Mills JC, Björkholm BM, Gordon JI. The impact of parietal cells on Helicobacter pylori tropism and host pathology: an analysis using gnotobiotic normal and transgenic mice. Proc Natl Acad Sci USA. 2003;100(6):3467–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, DeMatteo RP, Pamer EG. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455(7214):804–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Natividad JM, Petit V, Huang X, de Palma G, Jury J, Sanz Y, Philpott D, Garcia Rodenas CL, McCoy KD, Verdu EF. Commensal and probiotic bacteria influence intestinal barrier function and susceptibility to colitis in Nod1-/-; Nod2-/- mice. Inflamm Bowel Dis. 2012;18(8):1434–1446. [DOI] [PubMed] [Google Scholar]
- 36. Natividad JM, Hayes CL, Motta JP, Jury J, Galipeau HJ, Philip V, Garcia-Rodenas CL, Kiyama H, Bercik P, Verdu EF. Differential induction of antimicrobial REGIII by the intestinal microbiota and Bifidobacterium breve NCC2950. Appl Environ Microbiol. 2013;79(24):7745–7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Everard A, Lazarevic V, Gaïa N, Johansson M, Ståhlman M, Backhed F, Delzenne NM, Schrenzel J, François P, Cani PD. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014;8(10):2116–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hartmann P, Peng C, Wang HJ, Wang L, McCole DF, Brandi K, Stärkel P, Belzer C, Hellerbrand C, Tsukamoto H, Ho SB, Schnabl B. Deficiency of intestinal mucin‐2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013;58(1):108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53(1):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guerville M, Leroy A, Sinquin A, Laugerette F, Michalski MC, Boudry G. Western-diet consumption induces alteration of barrier function mechanisms in the ileum that correlates with metabolic endotoxemia in rats. Am J Physiol Endocrinol Metab. 2017;313(2):E107–E120. [DOI] [PubMed] [Google Scholar]
- 41. de Wit NJ, Bosch-Vermeulen H, de Groot PJ, Hooiveld GJ, Bromhaar MM, Jansen J, Müller M, van der Meer R. The role of the small intestine in the development of dietary fat-induced obesity and insulin resistance in C57BL/6J mice. BMC Med Genomics. 2008;1(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sala P, Torrinhas RS, Fonseca DC, Heymsfield S, Giannella-Neto D, Waitzberg DL. Type 2 diabetes remission after roux-en-Y gastric bypass: evidence for increased expression of jejunal genes encoding regenerating pancreatic islet-derived proteins as a potential mechanism. Obes Surg. 2017;27(4):1123–1127. [DOI] [PubMed] [Google Scholar]
- 43. Ben-Zvi D, Meoli L, Abidi WM, Nestoridi E, Panciotti C, Castillo E, Pizarro P, Shirley E, Gourash WF, Thompson CC, Munoz R, Clish CB, Anafi RC, Courcoulas AP, Stylopoulos N. Time-dependent molecular responses differ between gastric bypass and dieting but are conserved across species. Cell Metab. 2018;28(2):310–323.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao D, Kim YH, Jeong S, Greenson JK, Chaudhry MS, Hoepting M, Anderson ER, van den Brink MR, Peled JU, Gomes AL, Slingerland AE, Donovan MJ, Harris AC, Levine JE, Ozbek U, Hooper LV, Stappenbeck TS, Ver Heul A, Liu TC, Reddy P, Ferrara JL. Survival signal REG3α prevents crypt apoptosis to control acute gastrointestinal graft-versus-host disease. J Clin Invest. 2018;128(11):4970–4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ferrara JL, Harris AC, Greenson JK, Braun TM, Holler E, Teshima T, Levine JE, Choi SW, Huber E, Landfried K, Akashi K, Vander Lugt M, Reddy P, Chin A, Zhang Q, Hanash S, Paczesny S. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. 2011;118(25):6702–6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koehler JA, Baggio LL, Cao X, Abdulla T, Campbell JE, Secher T, Jelsing J, Larsen B, Drucker DJ. Glucagon-like peptide-1 receptor agonists increase pancreatic mass by induction of protein synthesis. Diabetes. 2015;64(3):1046–1056. [DOI] [PubMed] [Google Scholar]
- 47. Tremblay S, Romain G, Roux M, Chen XL, Brown K, Gibson DL, Ramanathan S, Menendez A. Bile acid administration elicits an intestinal antimicrobial program and reduces the bacterial burden in two mouse models of enteric infection. Infect Immun. 2017;85(6):e00942-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lai Y, Li D, Li C, Muehleisen B, Radek KA, Park HJ, Jiang Z, Li Z, Lei H, Quan Y, Zhang T, Wu Y, Kotol P, Morizane S, Hata TR, Iwatsuki K, Tang C, Gallo RL. The antimicrobial protein REG3A regulates keratinocyte proliferation and differentiation after skin injury. Immunity. 2012;37(1):74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wu Y, Quan Y, Liu Y, Liu K, Li H, Jiang Z, Zhang T, Lei H, Radek KA, Li D, Wang Z, Lu J, Wang W, Ji S, Xia Z, Lai Y. Hyperglycaemia inhibits REG3A expression to exacerbate TLR3-mediated skin inflammation in diabetes. Nat Commun. 2016;7(1):13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gironella M, Calvo C, Fernández A, Closa D, Iovanna JL, Rosello-Catafau J, Folch-Puy E. Reg3β deficiency impairs pancreatic tumor growth by skewing macrophage polarization. Cancer Res. 2013;73(18):5682–5694. [DOI] [PubMed] [Google Scholar]
- 51. Gironella M, Iovanna JL, Sans M, Gil F, Peñalva M, Closa D, Miquel R, Piqué JM, Panés J. Anti-inflammatory effects of pancreatitis associated protein in inflammatory bowel disease. Gut. 2005;54(9):1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kobayashi S, Akiyama T, Nata K, Abe M, Tajima M, Shervani NJ, Unno M, Matsuno S, Sasaki H, Takasawa S, Okamoto H. Identification of a receptor for reg (regenerating gene) protein, a pancreatic beta-cell regeneration factor. J Biol Chem. 2000;275(15):10723–10726. [DOI] [PubMed] [Google Scholar]
- 53. Oud MM, Tuijnenburg P, Hempel M, van Vlies N, Ren Z, Ferdinandusse S, Jansen MH, Santer R, Johannsen J, Bacchelli C, Alders M, Li R, Davies R, Dupuis L, Cale CM, Wanders RJA, Pals ST, Ocaka L, James C, Müller I, Lehmberg K, Strom T, Engels H, Williams HJ, Beales P, Roepman R, Dias P, Brunner HG, Cobben JM, Hall C, Hartley T, Le Quesne Stabej P, Mendoza-Londono R, Davies EG, de Sousa SB, Lessel D, Arts HH, Kuijpers TW. Mutations in EXTL3 cause neuro-immuno-skeletal dysplasia syndrome. Am J Hum Genet. 2017;100(2):281–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim BT, Kitagawa H, Tamura J, Saito T, Kusche-Gullberg M, Lindahl U, Sugahara K. Human tumor suppressor EXT gene family members EXTL1 and EXTL3 encode alpha 1,4- N-acetylglucosaminyltransferases that likely are involved in heparan sulfate/ heparin biosynthesis. Proc Natl Acad Sci USA. 2001;98(13):7176–7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Takahashi I, Noguchi N, Nata K, Yamada S, Kaneiwa T, Mizumoto S, Ikeda T, Sugihara K, Asano M, Yoshikawa T, Yamauchi A, Shervani NJ, Uruno A, Kato I, Unno M, Sugahara K, Takasawa S, Okamoto H, Sugawara A. Important role of heparan sulfate in postnatal islet growth and insulin secretion. Biochem Biophys Res Commun. 2009;383(1):113–118. [DOI] [PubMed] [Google Scholar]
- 56. Mueller CM, Zhang H, Zenilman ME. Pancreatic reg I binds MKP-1 and regulates cyclin D in pancreatic-derived cells. J Surg Res. 2008;150(1):137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Levetan CS, Upham LV, Deng S, Laury-Kleintop L, Kery V, Nolan R, Quinlan J, Torres C, El-Hajj RJ. Discovery of a human peptide sequence signaling islet neogenesis. Endocr Pract. 2008;14(9):1075–1083. [DOI] [PubMed] [Google Scholar]
- 58. Liu X, Zhou Z, Cheng Q, Wang H, Cao H, Xu Q, Tuo Y, Jiang L, Zou Y, Ren H, Xiang M. Acceleration of pancreatic tumorigenesis under immunosuppressive microenvironment induced by Reg3g overexpression. Cell Death Dis. 2017;8(9):e3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xia F, Cao H, Du J, Liu X, Liu Y, Xiang M. Reg3g overexpression promotes β cell regeneration and induces immune tolerance in nonobese-diabetic mouse model. J Leukoc Biol. 2016;99(6):1131–1140. [DOI] [PubMed] [Google Scholar]
- 60. Wang L, Fouts DE, Stärkel P, Hartmann P, Chen P, Llorente C, DePew J, Moncera K, Ho SB, Brenner DA, Hooper LV, Schnabl B. Intestinal REG3 lectins protect against alcoholic steatohepatitis by reducing mucosa-associated microbiota and preventing bacterial translocation. Cell Host Microbe. 2016;19(2):227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ampo K, Suzuki A, Konishi H, Kiyama H. Induction of pancreatitis-associated protein (PAP) family members in neurons after traumatic brain injury. J Neurotrauma. 2009;26(10):1683–1693. [DOI] [PubMed] [Google Scholar]
- 62. Kawahara S, Konishi H, Morino M, Ohata K, Kiyama H. Pancreatitis-associated protein-I and pancreatitis-associated protein-III expression in a rat model of kainic acid-induced seizure. Neuroscience. 2011;175:273–280. [DOI] [PubMed] [Google Scholar]
- 63. Loonen LM, Stolte EH, Jaklofsky MT, Meijerink M, Dekker J, van Baarlen P, Wells JM. REG3γ-deficient mice have altered mucus distribution and increased mucosal inflammatory responses to the microbiota and enteric pathogens in the ileum. Mucosal Immunol. 2014;7(4):939–947. [DOI] [PubMed] [Google Scholar]
- 64. Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334(6053):255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Choi SM, McAleer JP, Zheng M, Pociask DA, Kaplan MH, Qin S, Reinhart TA, Kolls JK. Innate Stat3-mediated induction of the antimicrobial protein Reg3γ is required for host defense against MRSA pneumonia. J Exp Med. 2013;210(3):551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lörchner H, Pöling J, Gajawada P, Hou Y, Polyakova V, Kostin S, Adrian-Segarra JM, Boettger T, Wietelmann A, Warnecke H, Richter M, Kubin T, Braun T. Myocardial healing requires Reg3β-dependent accumulation of macrophages in the ischemic heart. Nat Med. 2015;21(4):353–362. [DOI] [PubMed] [Google Scholar]
- 67. Hervieu V, Christa L, Gouysse G, Bouvier R, Chayvialle JA, Bréchot C, Scoazec JY. HIP/PAP, a member of the reg family, is expressed in glucagon-producing enteropancreatic endocrine cells and tumors. Hum Pathol. 2006;37(8):1066–1075. [DOI] [PubMed] [Google Scholar]
- 68. Gironella M, Folch-Puy E, LeGoffic A, Garcia S, Christa L, Smith A, Tebar L, Hunt SP, Bayne R, Smith AJ, Dagorn JC, Closa D, Iovanna JL. Experimental acute pancreatitis in PAP/HIP knock-out mice. Gut. 2007;56(8):1091–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Luo C, Yu LT, Yang MQ, Li X, Zhang ZY, Alfred MO, Liu JL, Wang M. Recombinant Reg3β protein protects against streptozotocin-induced β-cell damage and diabetes. Sci Rep. 2016;6(1):35640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Simpson RJ, Deenmamode J, McKie AT, Raja KB, Salisbury JR, Iancu TC, Peters TJ. Time-course of iron overload and biochemical, histopathological and ultrastructural evidence of pancreatic damage in hypotransferrinaemic mice. Clin Sci (Lond). 1997;93(5):453–462. [DOI] [PubMed] [Google Scholar]
- 71. Coffey R, Nam H, Knutson MD. Microarray analysis of rat pancreas reveals altered expression of Alox15 and regenerating islet-derived genes in response to iron deficiency and overload. PLoS One. 2014;9(1):e86019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Backe MB, Moen IW, Ellervik C, Hansen JB, Mandrup-Poulsen T. Iron regulation of pancreatic beta-cell functions and oxidative stress. Annu Rev Nutr. 2016;36(1):241–273. [DOI] [PubMed] [Google Scholar]
- 73. Yin G, Du J, Cao H, Liu X, Xu Q, Xiang M. Reg3g promotes pancreatic carcinogenesis in a murine model of chronic pancreatitis. Dig Dis Sci. 2015;60(12):3656–3668. [DOI] [PubMed] [Google Scholar]
- 74. Moniaux N, Song H, Darnaud M, Garbin K, Gigou M, Mitchell C, Samuel D, Jamot L, Amouyal P, Amouyal G, Bréchot C, Faivre J. Human hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein cures fas-induced acute liver failure in mice by attenuating free-radical damage in injured livers. Hepatology. 2011;53(2):618–627. [DOI] [PubMed] [Google Scholar]
- 75. Lieu HT, Batteux F, Simon MT, Cortes A, Nicco C, Zavala F, Pauloin A, Tralhao JG, Soubrane O, Weill B, Bréchot C, Christa L. HIP/PAP accelerates liver regeneration and protects against acetaminophen injury in mice. Hepatology. 2005;42(3):618–626. [DOI] [PubMed] [Google Scholar]
- 76. Simon MT, Pauloin A, Normand G, Lieu HT, Mouly H, Pivert G, Carnot F, Tralhao JG, Brechot C, Christa L. HIP/PAP stimulates liver regeneration after partial hepatectomy and combines mitogenic and anti-apoptotic functions through the PKA signaling pathway. FASEB J. 2003;17(11):1441–1450. [DOI] [PubMed] [Google Scholar]
- 77. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–1743. [DOI] [PubMed] [Google Scholar]
- 78. Baltzis D, Eleftheriadou I, Veves A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv Ther. 2014;31(8):817–836. [DOI] [PubMed] [Google Scholar]
- 79. Secq V, Mallmann C, Gironella M, Lopez B, Closa D, Garcia S, Christa L, Montalto G, Dusetti N, Iovanna JL. PAP/HIP protein is an obesogenic factor. J Cell Physiol. 2014;229(2):225–231. [DOI] [PubMed] [Google Scholar]
- 80. Hageman RS, Wagener A, Hantschel C, Svenson KL, Churchill GA, Brockmann GA. High-fat diet leads to tissue-specific changes reflecting risk factors for diseases in DBA/2J mice. Physiol Genomics. 2010;42(1):55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Huang Y, Qi H, Zhang Z, Wang E, Yun H, Yan H, Su X, Liu Y, Tang Z, Gao Y, Shang W, Zhou J, Wang T, Che Y, Zhang Y, Yang R. Gut REG3γ-associated Lactobacillus induces anti-inflammatory macrophages to maintain adipose tissue homeostasis. Front Immunol. 2017;8:1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bluemel S, Wang L, Martino C, Lee S, Wang Y, Williams B, Horvath A, Stadlbauer V, Zengler K, Schnabl B. The role of intestinal C-type regenerating islet derived-3 kectins for nonalcoholic steatohepatitis. Hepatol Commun. 2018;2(4):393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Miki T, Holst O, Hardt WD. The bactericidal activity of the C-type lectin RegIIIβ against Gram-negative bacteria involves binding to lipid A. J Biol Chem. 2012;287(41):34844–34855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14(3):282–289. [DOI] [PubMed] [Google Scholar]
- 85. Kinnebrew MA, Ubeda C, Zenewicz LA, Smith N, Flavell RA, Pamer EG. Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J Infect Dis. 2010;201(4):534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206(7):1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hanash AM, Dudakov JA, Hua G, O’Connor MH, Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, Ghosh A, Tsai JJ, Rao UK, Yim NL, Smith OM, Velardi E, Hawryluk EB, Murphy GF, Liu C, Fouser LA, Kolesnick R, Blazar BR, van den Brink MR. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37(2):339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Osman NM, Naora H, Otani H. Glycosyltransferase encoding gene EXTL3 is differentially expressed in the developing and adult mouse cerebral cortex. Brain Res Dev Brain Res. 2004;151(1-2):111–117. [DOI] [PubMed] [Google Scholar]
- 89. Saito T, Seki N, Yamauchi M, Tsuji S, Hayashi A, Kozuma S, Hori T. Structure, chromosomal location, and expression profile of EXTR1 and EXTR2, new members of the multiple exostoses gene family. Biochem Biophys Res Commun. 1998;243(1):61–66. [DOI] [PubMed] [Google Scholar]
- 90. Marchal S, Givalois L, Verdier JM, Mestre-Francés N. Distribution of lithostathine in the mouse lemur brain with aging and Alzheimer’s-like pathology. Neurobiol Aging. 2012;33(2):431.e15–431.e25. [DOI] [PubMed] [Google Scholar]
- 91. Arble DM, Sandoval DA, Seeley RJ. Mechanisms underlying weight loss and metabolic improvements in rodent models of bariatric surgery. Diabetologia. 2015;58(2):211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Makary MA, Clark JM, Shore AD, Magnuson TH, Richards T, Bass EB, Dominici F, Weiner JP, Wu AW, Segal JB. Medication utilization and annual health care costs in patients with type 2 diabetes mellitus before and after bariatric surgery [published correction appears in Arch Surg. 2011;146(6):659] Arch Surg. 2010;145(8):726–731. [DOI] [PubMed] [Google Scholar]
- 93. Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel-Courtin C, Gass M, Kern B, von Fluee M, Beglinger C. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22(5):740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hutch CR, Sandoval D. The role of GLP-1 in the metabolic success of bariatric surgery. Endocrinology. 2017;158(12):4139–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li JV, Ashrafian H, Bueter M, Kinross J, Sands C, le Roux CW, Bloom SR, Darzi A, Athanasiou T, Marchesi JR, Nicholson JK, Holmes E. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut. 2011;60(9):1214–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Guo Y, Liu CQ, Shan CX, Chen Y, Li HH, Huang ZP, Zou DJ. Gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in a diabetic rat model: increased diversity and associations of discriminant genera with metabolic changes. Diabetes Metab Res Rev. 2017;33(3):e2857. [DOI] [PubMed] [Google Scholar]
- 97. Myronovych A, Kirby M, Ryan KK, Zhang W, Jha P, Setchell KD, Dexheimer PJ, Aronow B, Seeley RJ, Kohli R. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring). 2014;22(2):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Evers SS, Sandoval DA, Seeley RJ. The physiology and molecular underpinnings of the effects of bariatric surgery on obesity and diabetes. Annu Rev Physiol. 2017;79(1):313–334. [DOI] [PubMed] [Google Scholar]
- 99. von Drygalski A, Andris DA. Anemia after bariatric surgery: more than just iron deficiency. Nutr Clin Pract. 2009;24(2):217–226. [DOI] [PubMed] [Google Scholar]
- 100. Stelter C, Käppeli R, König C, Krah A, Hardt WD, Stecher B, Bumann D. Salmonella-induced mucosal lectin RegIIIβ kills competing gut microbiota. PLoS One. 2011;6(6):e20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Darnaud M, Dos Santos A, Gonzalez P, Augui S, Lacoste C, Desterke C, De Hertogh G, Valentino E, Braun E, Zheng J, Boisgard R, Neut C, Dubuquoy L, Chiappini F, Samuel D, Lepage P, Guerrieri F, Doré J, Bréchot C, Moniaux N, Faivre J. Enteric delivery of regenerating family member 3 alpha alters the intestinal microbiota and controls inflammation in mice with colitis. Gastroenterology. 2018;154(4):1009–1023.e14. [DOI] [PubMed] [Google Scholar]