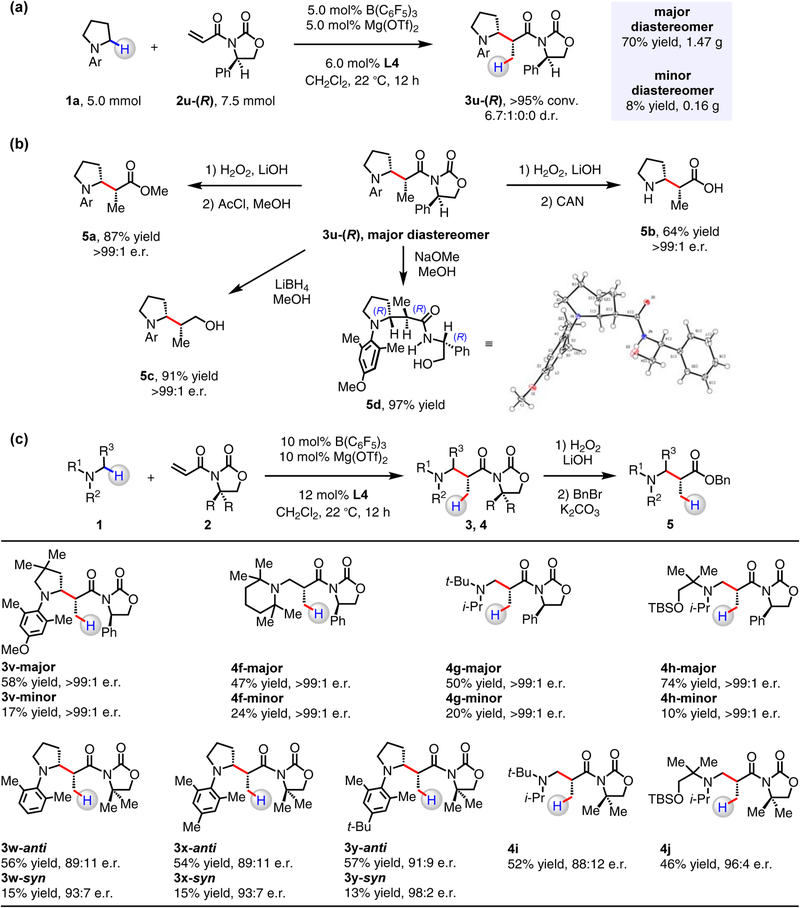
Figure 5. Synthesis of chiral α-substituted amines.
(a) As demonstrated through preparation of β-amino amide 3u-(R), the catalytic reactions are amenable to gram-scale operations. (b) The versatility of 3u-(R) was demonstrated by its conversion into a β-amino ester, a β-amino alcohol and a β-amino acid. The absolute configuration of 3u-(R) was determined by X-ray crystallographic analysis of 5d. (c) A series of N-alkylamines reacted with 2 to afford the corresponding β-amino amides. For 3v and 4f–4h, diastereomers were isolated by silica gel chromatography and then converted into β-amino esters (5) that were determined to be enantiomerically pure.