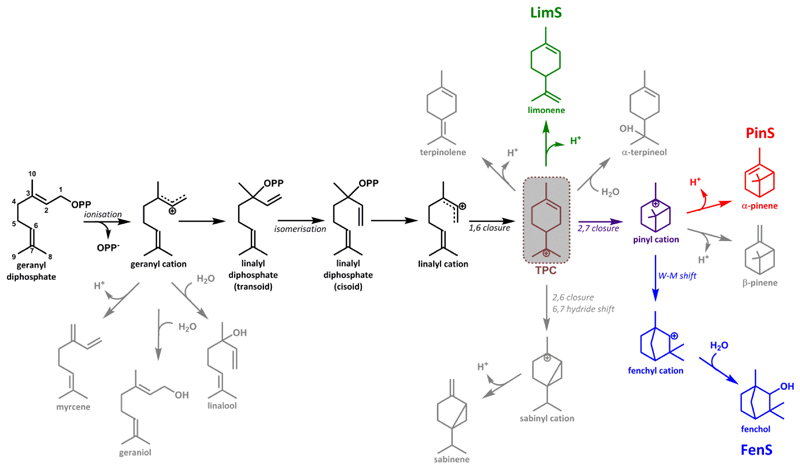
Figure 1. Proposed mechanism for the formation of limonene, pinene, fenchol and common by-products.
All mTC/S reactions commonly start with the ionization of the geranyl diphosphate (GPP) substrate resulting in the geranyl cation, which subsequently can undergo a wide range of cyclizations and rearrangements before the reaction is terminated by deprotonation or water attack. The formation of all cyclic products requires the isomerization of the geranyl cation to the linalyl cation via linalyl diphosphate. The latter can cyclize to yield the α-terpinyl cation (TPC, grey box). The main reaction cascades of LimS, PinS, and FenS following the formation of TPC are indicated in green, red and blue respectively. Premature quenching of intermediates may result in the formation of various by-products (grey). Carbon atom numbering of intermediates refers to that for the GPP substrate.