Table 2. Multi-step biocatalysis and chemo-enzymatic routes to industrially-useful compounds.
| Entry | Final product (s) | Uses | ER(s) | Other enz. | Ref. |
|---|---|---|---|---|---|
| 1 |
 4-methylheptan-3-ol1 |
Insect pest management | OYE2.6 OYE1 W116V | ADH440 ADH270 | 66 |
| 2 |
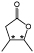 3-Methyl-4-pentanolide1 |
Fragrance industry | OYE2 | KRED READH | 67 |
| 3 |
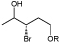 R=Ac, Bz, Bn |
Precursors of drugs, flavours, and agrochemicals | OYE3 | EVO030 EVO270 | 68 |
| 4 |
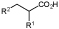 R1=Me, Ph, NHCHO; R2=Et, Ph, 4-Cl-Ph |
Cosmetics, food additives, and pharmaceuticals | OYE2 | EcAldDH | 12 |
| 5 |
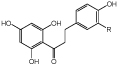 R=H, OH, OMe |
Antioxidants and flavor enhancers | sERED | CHI | 69 |
| 6 |
 Butanol |
Biofuel | YqjM | PDC, ADH | 70 |
| 7 |
 (R) and (S) forms |
Industrially-useful synthons | YqjM C26D/I69T variant | ATA | 71 |
Four stereoisomers produced.
Highlighted chiral centres. ER sources: OYE2.6 = P. stipitis; OYE1 = Saccharomyces pastorianus. Other enz = other biocatalysts utilised within the cascading reactions, excluding cofactor regenerating enzymes.
