Abstract
Background
Biliary atresia (BA) is a life-threatening liver disease of infancy, characterized by extrahepatic biliary obstruction, bile retention, and progressive liver injury. The Kasai portoenterostomy (KP) is BA's only nontransplant treatment. Its success is variable and depends on restoration of hepatic bile flow. Many adjunctive therapeutics have been studied to improve outcomes after the KP, but none demonstrate effectiveness. This study tests if N-acetylcysteine (NAC), a precursor to the choleretic glutathione, improves bile flow after KP.
Methods
This report describes the design of an open-label, single center, Phase 2 study to determine the effect of NAC following KP on markers of bile flow and outcomes in BA. The intervention is intravenous NAC (150 mg/kg/day) administered continuously for seven days starting 0–24 h after KP. The primary outcome is normalization of total serum bile acid (TSBA) concentrations within 24 weeks of KP. The secondary objectives are to describe NAC therapy's effect on other clinical parameters followed in BA for 24 months and to report adverse events occurring with therapy. This study follows the “minimax” clinical trial design.
Discussion
This is the first clinical trial to test NAC's effectiveness in improving bile flow after KP in BA. It introduces three important concepts for future BA therapeutic trials: (1) the “minimax” study design, a pertinent design for rare diseases because it detects potential effects quickly with small subject size; (2) the more sensitive bile flow marker, TSBAs, which may correlate with positive long-term outcomes better than traditional bile flow markers such as serum bilirubin; and (3) liver enzyme changes immediately after KP, which can be a guideline for potential drug-induced liver injury in other BA peri-operative adjunctive therapeutic trials.
Keywords: Biliary atresia, Kasai portoenterostomy, N-acetylcysteine, Minimax design, Serum bile acids, Drug-induced liver injury
Abbreviations: BA, Biliary atresia; KP, Kasai portoenterostomy; NAC, N-acetylcysteine; TSBA, Total serum bile acids; DILI, Drug-induced liver injury; START, Steroids in Biliary Atresia Randomized Trial; FDA, Food and Drug administration; DSMB, Data and Safety Monitoring Board; Bc, Conjugated bilirubin; AST, Aspartate aminotransferase; ALT, Alanine transaminase; GGT, Gamma-glutamlytransferase; TB, Total bilirubin; TCH, Texas Children's Hospital; IOC, Intraoperative cholangiogram; DoL, Day of life
1. Introduction
Biliary atresia (BA), a serious liver disease affecting 1 in 8000–18,000 infants, is characterized by extrahepatic bile duct obstruction impairing normal bile flow [1,2]. As a result, bile is retained in the liver, leading to progressive liver damage and eventually end-stage liver disease. To attempt to restore bile flow and slow disease progression, the Kasai portoenterostomy (KP) can be performed. The KP surgically removes the atretic bile ducts and connects the liver hilum directly to the intestines. Unfortunately, KPs have uneven success rates, with over 70% of infants still needing liver transplantation by adulthood for survival [3,4]. Approximately half of these children require a liver transplant before age two [5,6]. As a result, BA is the single most common indication for pediatric liver transplantation in the United States and worldwide [7].
While numerous medical therapies have been proposed to enhance KP outcomes, to date none have shown clear efficacy. For example, corticosteroids have been tested after KP, based on the assumption that bile duct obstruction occurs secondary to immune-mediated destruction of bile ducts. In the Steroids in Biliary Atresia Randomized Trial (START), a multi-center randomized double-blind placebo-controlled study of intravenous (IV) corticosteroids after KP, no difference in transplant-free survival or bilirubin clearance was seen in infants receiving steroids relative to placebo [8]. Intravenous immunoglobulins (IVIg) was also studied in a Phase 2 multi-center study, but showed no improvement in serum bilirubin clearance and 1-year transplant-free survival after KP compared to a historical control group [9]. Thus, there is a critical need of therapies to improve bile flow and outcomes after KP.
In this report, we describe a Phase 2 study testing N-acetylcysteine (NAC) in BA after KP. The choice of NAC is two-fold: (1) it is the precursor for glutathione, a stimulant of bile flow; and (2) it can be administered intravenously, immediately after the KP, to potentially help restore bile flow quickly when oral agents such as ursodeoxycholic acid cannot be given. Three areas of novelty are discussed: (1) applying the Phase 2 “minimax” trial design to test a BA therapeutic; (2) total serum bile acids (TSBAs) as an end-point in BA studies; and (3) trends in laboratory tests after KP, to help identify potential drug-induced liver injury (DILI).
2. Methods
2.1. Study oversight
The trial (NCT03499249) as well as the retrospective analysis to describe laboratory changes after KP have been approved by the Baylor College of Medicine Investigation Review Board. The principal investigator (S.H.) holds the investigational new drug application from the Food and Drug Administration (FDA) (IND #135796). Funding is from private groups (Baylor College of Medicine Nancy Chang, Ph.D. Award for Research Excellence and The Men of Distinction Pediatric Research Award), and NAC is provided by the manufacturer (Cumberland Pharmaceuticals, Nashville, TN). The trial opened for enrollment in September 2017. Trial oversight is provided by a Data and Safety Monitoring Board (DSMB) comprised of experts in pediatric hepatology and pharmacology from institutions outside the study site. No DSMB member has any conflicts of interest with the study.
2.2. Study overview and endpoints
This report describes an open-label, single center, Phase 2 study to determine if seven days of intravenous NAC treatment immediately after KP improves surrogate markers of bile flow and outcomes in BA. The primary endpoint is achieving normal TSBAs (≤10 μmol/L) within the first 24 weeks after KP. The secondary endpoints are: (1) changes in laboratory markers, including markers of liver damage and function (TSBAs, conjugated bilirubin [Bc], aspartate aminotransferase [AST], alanine aminotransferase [ALT], gamma-glutamyltransferase [GGT], albumin, sodium, total bilirubin [TB], platelets, INR), in the first two years of life; (2) growth and nutritional status in the first two years of life; (3) occurrence of sentinel events related to worsening liver disease (cholangitis, development of ascites, variceal bleeding, liver transplant listing, liver transplantation and/or death) in the first two years of life; and (4) occurrence of adverse events which may be associated with NAC therapy.
2.3. Participant selection
All infants with suspected BA at Texas Children's Hospital (TCH) are considered for enrollment if they meet the study's inclusion and exclusion criteria (Table 1). Inclusion criteria include a BA diagnosis made by the gold-standard intraoperative cholangiogram (IOC) and KP performed before 90 days of life (DoL). Exclusion criteria include evidence of early synthetic liver dysfunction from rapidly progressive disease, infection, severe acute kidney injury, and other co-morbidities which would interfere with the conduct and results of the study. For potentially eligible infants, parent(s)/guardian(s) are approached before the IOC/KP is performed and information about the trial is shared. If the IOC confirms the BA diagnosis, informed consent is obtained within 24 h after KP.
Table 1.
Inclusion and exclusion criteria.
| Inclusion Criteria (all must be met) |
|---|
|
|
|
| Exclusion Criteria |
|
|
|
|
2.4. Study treatment and concurrent treatments
Intravenous NAC (150 mg/kg/day) is administered continuously, starting 0–24 h after KP and lasting for seven days. To achieve a continuous infusion, a working solution of 10 mg/mL (382 mOsm) is created following standard pharmacy protocols, using a 200 mg/mL (2600 mOsm) stock solution of Acetadote (Cumberland Pharmaceuticals, Nashville, TN) diluted with 5% dextrose. In addition, all routine therapies including ursodeoxycholic acid, fat-soluble vitamins, and antibiotics are given post-KP according to previously published approaches [8]. No corticosteroids are administered post-KP. In addition, no other investigational therapies are given during the study period.
2.5. Conduct of trial
This trial involves NAC treatments during the post-operative course after KP as well as standard of care visits to TCH after KP. At TCH, the typical post-operative admission time after KP is 7–10 days, which is enough time for NAC intravenous therapy to finish. The post-operative outpatient visits are scheduled at 14 days after KP; 1, 2, 3, 4.5, and 6 months after KP; and 12, 18, and 24 months of life. Data collection occurs at these schedule times, using clinical information and laboratory test results collected routinely during clinical care at TCH including TSBAs (Table 2). In addition to the visits, more frequent inpatient and outpatient visits may be performed as needed, depending on each participant's clinical course after KP.
Table 2.
Trial observations.
| Pre-KPa | Days Post-KP |
Weeks Post-KP |
Months of Life |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3b | 7 | 10 | 2 | 4 | 8 | 12 | 18 | 24 | 12 | 18 | 24 | ||
| Demographic data | ● | ||||||||||||
| TSBAs | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||
| Bc | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| AST | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| ALT | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| GGT | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Albumin | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Sodium | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Total Bilirubin | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Platelets | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||
| INR | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||
| 25-hydroxy Vitamin D | ● | ● | ● | ● | ● | ● | ● | ||||||
| Vitamin A | ● | ● | ● | ● | ● | ● | ● | ||||||
| Vitamin E | ● | ● | ● | ● | ● | ● | ● | ||||||
| Weight | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Adverse events | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |
Drawn within seven days of KP.
If laboratory adverse event occurs (see Table 3), will perform an additional measurement 5 days post-KP.
2.6. Sample size calculations
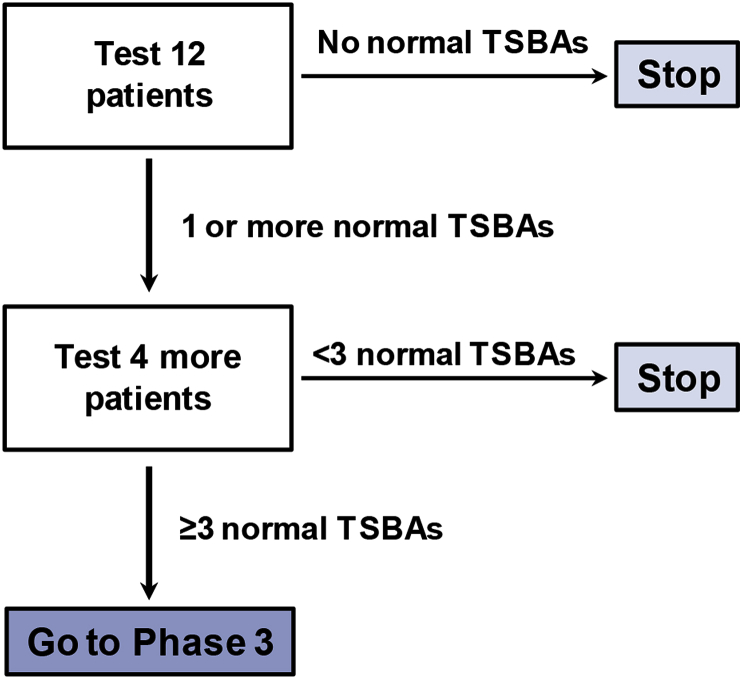
This trial uses the two-stage “minimax” design powered for the primary endpoint [10]. The null hypothesis is that NAC has no effect and that 5% of infants post-KP will achieve normal TSBAs post-KP, the current expected clinical course (see Results/Discussion for assumptions about normal TSBAs after KP). The null hypothesis is tested against the alternative hypothesis that NAC increases the number of infants achieving normal TSBAs to 25%. In the first stage, 12 participants will be accrued. If no participant achieves TSBAs ≤10 μmol/L within 24 weeks post-KP, the study will be stopped. Otherwise, four additional infants will be accrued for a total of 16 participants. The null hypothesis will be rejected if ≥ 3 of 16 participants achieve TSBAs ≤10 μmol/L within 24 weeks post-KP. This design yields a type I error rate of 5% and power of 80% when the true response rate is 25%. This study is not powered for secondary endpoints.
2.7. Stopping rules
Criteria for stopping NAC infusions are borrowed from previous studies of NAC in infants and children [[11], [12], [13], [14]]. Briefly, the infusion will continue if flushing alone occurs; the infusion will be stopped if urticaria develops and restarted if responsive to diphenhydramine within 1 h; and will be stopped permanently if signs of anaphylaxis such as angioedema, hypotension, or wheezing develop, regardless of response to therapy. For the peri-operative period of BA specifically, additional stoppage criteria include elevation of alanine aminotransferase (ALT) > 700 IU/L above baseline, conjugated bilirubin (Bc) three times above baseline, Bc > 5 mg/dL above baseline, and/or total bilirubin (TB) three times above baseline (see Results/Discussion for derivation of these cut-off values) (Table 3). Finally, if an unexpected severe adverse event that may be related to NAC occurs any time during the NAC infusion, the trial will be paused until further guidance by the DSMB.
Table 3.
Laboratory parameters for possible DILI Post-KP.
| Adverse Event (results in enhanced monitoring) | |
|
|
|
|
|
|
| Serious Adverse Event (results in stopping infusion) | |
|
|
|
|
|
|
|
|
Values closest to time of KP within seven days of the procedure.
3. Results/discussion
3.1. NAC as a BA therapeutic
This study tests NAC as a therapeutic for BA after KP. NAC is the precursor for glutathione, a major component of human bile and based on rodent studies, a potent stimulant of bile flow [[15], [16], [17], [18]]. Our hypothesis is that immediately after the KP, stimulants of bile flow can improve success by establishing early bile flow out of the newly formed liver-intestine anastomosis and thereby preventing further obstruction and injury. There are at least two other determinants of bile flow in addition to glutathione, the major of which are bile acids and bicarbonate secretion from bile ducts. Bile acids are supplemented via oral administration of ursodeoxycholic acid, and bicarbonate secretion is induced by feeding. However, unlike intravenous NAC, to utilize these two other bile flow determinants, they must be given into a functioning intestine, which may occur only after many days post-KP. As a result, this trial administers NAC for the first seven days after KP, to bridge the period when ursodeoxycholic acid or feeding cannot be given.
NAC and its product glutathione may have other beneficial properties in BA in addition to stimulating bile flow. Glutathione has well-characterized anti-oxidant properties, which could scavenge free radicals that may contribute to cirrhosis [18]. Glutathione is also the natural substrate for GGT, an enzyme expressed by hepatocytes and cholangiocytes [15,[19], [20], [21]]. GGT is often upregulated in BA, so providing more glutathione could potentially serve a nutritive role for cholangiocytes. Consistent with these possible beneficial effects, in several pre-clinical studies, NAC administered to rodents with cholestasis decreased inflammatory cytokines and markers of liver fibrosis [18,22]. Similarly, in a toxin-induced zebrafish model of BA, NAC was able to increase glutathione levels in cholangiocytes and decrease biliary damage [23,24].
Importantly, intravenous NAC has been tested in numerous pediatric studies and has an excellent safety record (Table 4). Many of these reports involve administering NAC for acetaminophen toxicity, NAC's FDA-approved indication. Other studies involve administering NAC for other forms of liver disease. Squires et al. tested up to seven days of intravenous NAC (150 mg/kg/day) in infants and children with non-acetaminophen induced acute liver failure, a disease group encompassing a broad set of conditions with different pathophysiological mechanisms than BA [14]. In these studies, NAC was not given as a choleretic agent; rather, NAC was hypothesized to possibly have a liver protective effect based on studies in adults with non-acetaminophen induced liver failure (the trial actually found that NAC therapy associated with greater need for transplant in this specific pediatric population). The trial reported no adverse events associated with continuous intravenous infusion of NAC in children. A lack of adverse events was also reported in studies administering NAC to vulnerable infant populations, including premature infants, newborns with neonatal hemochromatosis/gestational alloimmune liver disease, and parenteral nutrition-associated cholestasis [[25], [26], [27]].
Table 4.
Other neonatal diseases treated with IV NAC.
One concern is that NAC may have a different safety profile in the post-operative setting. However, multiple small trials testing NAC after cardiopulmonary bypass suggest it would be safe to use post-KP. For example, intravenous NAC was given to adults peri-operatively during abdominal aortic aneurysm repair and continued 24 h post-operatively. In this double-blind, randomized placebo-controlled trial, no reported adverse events were associated with NAC therapy [31]. A second similar double-blind randomized control trial for NAC during coronary artery bypass surgery showed a comparable safety profile [32].
3.2. Phase 2 “minimax” trial design
This trial uses the two-stage “minimax” design originally described by Simon, a design commonly used in oncological trials to determine whether a particularly therapy has sufficient therapy to warrant a larger Phase 3 trial [10]. The experience from this trial could serve as a model to help guide how BA therapeutic trials designs in the future. Two qualities make this approach useful for rare diseases such as BA: (1) early termination if the drug is not apparently efficacious, and (2) use of historical controls which simplifies study execution, enhances acceptability, and reduces the overall sample size required. In this study, enrollment stops after 12 participants if there is not a sufficient response in Stage 1. If there is sufficient response, enrollment will stop after only 16 infants are enrolled (Fig. 1).
Fig. 1.
Trial Design. This is a two-stage Phase 2 trial based on the “minimax” design. In Stage 1, 12 patients will be enrolled. If no patient in Stage 1 achieves a TSBA ≤10 μmol/L, the trial will stop. If at least one patient achieves a normal TSBA, an additional four patients will be enrolled. The trial is designed to detect a change in response rate from 5% without NAC treatment (based on historical controls) to 25% with NAC treatment, with a type I error rate of 5% and power of 80%.
Importantly, the Phase 2 “minimax” design does have limitations. First, it uses historical controls for comparison, thereby risking a type I error for those diseases whose management has improved over time. However, for BA, this is less of a concern because management has centered on the KP since the 1950s and outcomes have changed little in the United States over the last two decades. Second, the study design only identifies large effects (response improvements >20%) and may miss modestly efficacious therapies. For BA this is acceptable, because the field needs robust therapies that can substantially limit liver damage and delay/prevent need for transplantation. Third, the study design does not prove a therapy is efficacious. Rather, it quickly identifies those most worthy for larger, more expensive, and more time-consuming Phase 3 trials.
3.3. TSBAs as an end-point in BA
Rather than the TSBAs used in this study, most BA studies use serum TB as a measure of bile flow and endpoint after KP. For example, in the START trial, achieving a TB < 1.5 mg/dL within six-months post-KP was defined as “successful” [8]. Similarly, Shneider et al. found that achieving a TB < 2 mg/dL within three-months post-KP predicted better two-year transplant-free survival [33]. However, many infants with normal TB levels still have evidence of impaired bile flow and progressive liver disease, thus limiting the use of TB as a marker for bile flow. For example, as many as 45% of infants with TB < 2 mg/dL within three-months post-KP still developed portal hypertension, as indicated by thrombocytopenia [33].
This study uses a much stricter surrogate of bile flow—normalization of TSBAs to ≤10 μmol/L—as an endpoint. TSBAs can vary widely after KP even after serum bilirubin levels normalize [34]. In contrast, TSBA normalization is rare. For example, in a study comparing fat-soluble vitamin levels to bilirubin levels and TSBAs after KP, only approximately 5% of infants had TSBA levels ≤10 μmol/L [35]. These lower TSBAs in turn correlate with better long term outcomes: in a study of infants with normal serum bilirubin after KP, those with lower TSBAs had less evidence of liver injury and portal hypertension at two years. Furthermore, all infants with lower TSBAs by 6 months post-KP survived transplant-free during the study's 11 year follow-up [34].
3.4. DILI versus normal changes in the peri-operative period after KP
This study proposes laboratory guidelines for DILI after KP. To date, such parameters do not exist, in part because patients with BA already have elevated liver enzymes before KP or other experimental therapy. For example, baseline AST, ALT, GGT, Bc, and TB in infants with BA are already classified as National Cancer Institute-Common Terminology Criteria for Adverse Events (CTCAE) Grade 3. Hence, it is difficult to attribute worsening of laboratory values after KP to an experimental therapy or simply the natural post-operative course.
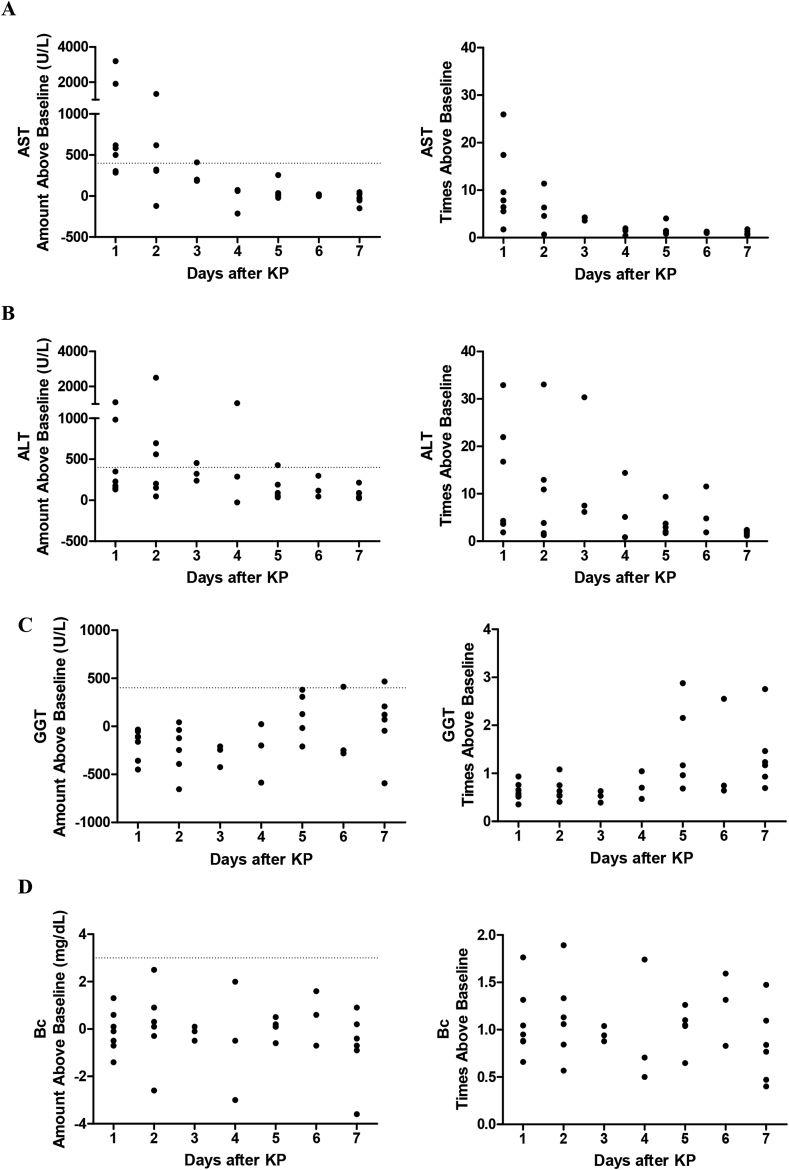
To better address this issue, we sought to first understand the natural history of BA in the immediate post-operative period. Laboratory values measured pre-KP (latest value drawn within seven days pre-KP) were compared with those drawn in the first seven days post-KP. There were 13 patients included in this analysis, all who underwent KP at our institution and did not receive any additional therapies. Ten of 13 patients (76.9%) survived transplant free at the two year mark. Immediately after KP, highest AST levels were >3000 U/L above baseline (>25 times baseline) and then dropped over time (Fig. 2a). The highest ALT levels were seen two days after KP, and similar to AST, dropped in subsequent days (Fig. 2b). GGT levels remained near or below baseline for the first four days, after which some GGT levels exceeded baseline values (Fig. 2c). Finally, Bc values varied, with some slight deviations above and below baseline in the first seven days after KP (Fig. 2d).
Fig. 2.
Liver Enzyme Changes in the First Seven Days after KP. Thirteen infants undergoing KP had laboratory values followed in the first seven post-operative days. (A and B) AST and ALT rose substantially above baseline on post-operative day 1 and then trended downwards. (C) GGT rose above baseline 5–7 days post-operatively. (D) Bc remained close to baseline in the immediate post-operative period. Dashed lines denote proposed upper limits for DILI.
Given these changes, DILI guidelines were proposed in conjunction with the FDA while the trial's IND application was under consideration. Parameters were also estimated for TB, though TB was not directly measured in the initial group of patients. The first criteria are defined as “adverse events,” which are monitored from the first laboratory measurements (post-operative day 3) and reported during the conduct of the trial (Table 3). They include increases in AST, ALT, and/or GGT >400 U/L above pre-KP values, an increase in Bc > 3 mg/dL above pre-KP values, or an increase in TB > 5 mg/dL above pre-KP values. If a laboratory adverse event does occur on the first measurement, an additional measurement at five days post-KP will be performed to ensure closer monitoring (Table 2). The second criteria are defined as “serious adverse events,” which necessitate stopping the NAC infusion and following participants off therapy until laboratory values stabilize or resolve. They include increases in ALT >700 U/L above pre-KP values, increase in Bc > 5 mg/dL or 3 times above pre-KP values, or increase in TB 3 times above pre-KP values. The parameters derived in this study can be modified and adopted by other investigators and the FDA to develop DILI definitions and potential stopping rules in BA peri-operative adjunctive therapeutic trials.
4. Conclusion/summary
This Phase 2 study tests intravenous NAC in BA after KP. The trial capitalizes on NAC's potential to stimulate bile flow and its ability to be delivered immediately post-operatively at a time when other stimulants of bile flow, such as bile acids and feeding, cannot be given. The trial introduces several innovative concepts, including (1) testing BA therapeutics with a two-stage “minimax” trial design; (2) using TSBAs as an endpoint for successful KP; and (3) establishing DILI guidelines for post-KP adjunctive therapeutic trials in BA, based on laboratory changes that normally occur after KP.
Trial registration
Declarations
Not applicable.
Ethics approval and consent to participate
Granted by Baylor College of Medicine Institutional Review Board.
Consent for publication
Not applicable.
Conflicts of interest
Not applicable.
Funding
Baylor College of Medicine Nancy Chang, Ph.D. Award for Research Excellence; Men of Distinction Pediatric Research Award; NIH 1 K23 DK109207.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
MET, BLS, and SH prepared the manuscript and analyzed data. BLS, MLB, DNC, and SH designed the study and developed the trial protocol. All authors read and approved the final manuscript.
Contributor Information
Mary Elizabeth M. Tessier, Email: metessie@bcm.edu.
Benjamin L. Shneider, Email: benjamin.shneider@bcm.edu.
Mary L. Brandt, Email: mary.brandt@bcm.edu.
Dana N. Cerminara, Email: dncermin@texaschildrens.org.
Sanjiv Harpavat, Email: harpavat@bcm.edu.
References
- 1.Jimenez-Rivera C., Jolin-Dahel K.S., Fortinsky K.J., Gozdyra P., Benchimol E.I. International incidence and outcomes of biliary atresia. J. Pediatr. Gastroenterol. Nutr. 2013;56:344–354. doi: 10.1097/MPG.0b013e318282a913. [DOI] [PubMed] [Google Scholar]
- 2.Bezerra J.A., Wells R.G., Mack C.L., Karpen S.J., Hoofnagle J.H., Doo E., Sokol R.J. BILIARY ATRESIA: clinical and Research challenges for the 21st century. Hepatology. 2018 doi: 10.1002/hep.29905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bezerra J.A., Wells R.G., Mack C.L., Karpen S.J., Hoofnagle J.H., Doo E., Sokol R.J. BILIARY ATRESIA: Clinical and Research Challenges for the 21 St Century. Hepatology. 2018 doi: 10.1002/hep.29905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sundaram S.S., Mack C.L., Feldman A.G., Sokol R.J. Biliary atresia: indications and timing of liver transplantation and optimization of pretransplant care. Liver Transplant. 2017;23:96–109. doi: 10.1002/lt.24640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shneider B.L., Brown M.B., Haber B., Whitington P.F., Schwarz K., Squires R., Bezerra J., Shepherd R., Rosenthal P., Hoofnagle J.H., Sokol R.J. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J. Pediatr. 2006;148:467–474. doi: 10.1016/j.jpeds.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 6.Davenport M., Caponcelli E., Livesey E., Hadzic N., Howard E. Surgical outcome in biliary atresia. Ann. Surg. 2008;247:694–698. doi: 10.1097/SLA.0b013e3181638627. [DOI] [PubMed] [Google Scholar]
- 7.Squires R.H., Ng V., Romero R., Ekong U., Hardikar W., Emre S., Mazariegos G.V. Evaluation of the pediatric patient for liver transplantation: 2014 practice guideline by the american association for the study of liver diseases, american society of transplantation and the north american society for pediatric gastroenterology, hepatolo. Hepatology. 2014;60:362–398. doi: 10.1002/hep.27191. [DOI] [PubMed] [Google Scholar]
- 8.Bezerra J.A., Spino C., Magee J.C., Shneider B.L., Rosenthal P., Wang K.S., Erlichman J., Haber B., Hertel P.M., Karpen S.J., Kerkar N., Loomes K.M., Molleston J.P., Murray K.F., Romero R., Schwarz K.B., Shepherd R., Suchy F.J., Turmelle Y.P., Whitington P.F., Moore J., Sherker A.H., Robuck P.R., Sokol R.J. Use of corticosteroids after hepatoportoenterostomy for bile drainage in infants with biliary atresia: the START randomized clinical trial. J. Am. Med. Assoc. 2014;311:1750–1759. doi: 10.1001/jama.2014.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sokol C.L., Ronald J., Spino Cathie, Moore Jeffrey, Bezerra Jorge, Whitington Peter F., Karpen Saul J., Loomes Kathleen M., Ng Vicky L., Venkat Veena L., Wang Kasper S., Goodhue Catherine J., Sherker Averell H., Magee John C., Mack Intravenous immunoglobulin (IVIG) following portoenterostomy in infants with biliary atresia: a phase 1/2a trial. Hepatology. 2016;64:1123A. [Google Scholar]
- 10.Simon R. Optimal two-stage designs for phase II clinical trials., Control. Clin. Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins D.D., Wiest D.B., Mulvihill D.M., Hlavacek A.M., Majstoravich S.J., Brown T.R., Taylor J.J., Buckley J.R., Turner R.P., Rollins L.G., Bentzley J.P., Hope K.E., Barbour A.B., Lowe D.W., Martin R.H., Chang E.Y. Fetal and neonatal effects of N-acetylcysteine when used for neuroprotection in maternal chorioamnionitis. J. Pediatr. 2016;168 doi: 10.1016/j.jpeds.2015.09.076. 67–76.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kortsalioudaki C., Taylor R.M., Cheeseman P., Bansal S., Mieli-vergani G., Dhawan A. Safety and efficacy of N-acetylcysteine in children with non-acetaminophen-induced acute liver failure. Liver Transplant. 2008;44:25–30. doi: 10.1002/lt.21246. [DOI] [PubMed] [Google Scholar]
- 13.Wiest D.B., Chang E., Fanning D., Garner S., Cox T., Jenkins D.D. Antenatal pharmacokinetics and placental transfer of N-acetylcysteine in chorioamnionitis for fetal neuroprotection. J. Pediatr. 2014;165:672–677.e2. doi: 10.1016/j.jpeds.2014.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Squires R.H., Dhawan A., Alonso E., Narkewicz M.R., Shneider B.L., Rodriguez-Baez N., Olio D.D., Karpen S., Bucuvalas J., Lobritto S., Rand E., Rosenthal P., Horslen S., Ng V., Subbarao G., Kerkar N., Rudnick D., Lopez M.J., Schwarz K., Romero R., Elisofon S., Doo E., Robuck P.R., Lawlor S., Belle S.H. Intravenous N-acetylcysteine in pediatric patients with nonacetaminophen acute liver failure: a placebo-controlled clinical trial. Hepatology. 2013;57:1542–1549. doi: 10.1002/hep.26001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballatori N., Jacob R., Boyer J.L. Intrabiliary glutathione hydrolysis. A source of glutamate in bile. J. Biol. Chem. 1986;261:7860–7865. [PubMed] [Google Scholar]
- 16.Tahan G., Tarcin O., Tahan V., Eren F., Gedik N., Sahan E., Biberoglu N., Guzel S., Bozbas A., Tozun N., Yucel O. The effects of N-acetylcysteine on bile duct ligation–induced liver fibrosis in rats. Dig. Dis. Sci. 2007;52:3348–3354. doi: 10.1007/s10620-006-9717-9. [DOI] [PubMed] [Google Scholar]
- 17.Galicia-Moreno M., Rodríguez-Rivera A., Reyes-Gordillo K., Segovia J., Shibayama M., Tsutsumi V., Vergara P., Moreno M.G., Muriel P. N-acetylcysteine prevents carbon tetrachloride-induced liver cirrhosis: role of liver transforming growth factor-beta and oxidative stress. Eur. J. Gastroenterol. Hepatol. 2009;21:908–914. doi: 10.1097/MEG.0b013e32831f1f3a. [DOI] [PubMed] [Google Scholar]
- 18.Galicia-Moreno M., Favari L., Muriel P. Antifibrotic and antioxidant effects of N-acetylcysteine in an experimental cholestatic model. Eur. J. Gastroenterol. Hepatol. 2012;24:179–185. doi: 10.1097/MEG.0b013e32834f3123. [DOI] [PubMed] [Google Scholar]
- 19.Hanigan M.H., Ricketts W.A. Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase. Biochemistry. 1993;32:6302–6306. doi: 10.1021/bi00075a026. http://www.ncbi.nlm.nih.gov/pubmed/8099811 accessed. [DOI] [PubMed] [Google Scholar]
- 20.Ballatori N., Jacob R., Barrett C., Boyer J.L. Biliary catabolism of glutathione and differential reabsorption of its amino acid constituents. Am. J. Physiol. 1988;254:G1–G7. doi: 10.1152/ajpgi.1988.254.1.G1. [DOI] [PubMed] [Google Scholar]
- 21.Hanigan M.H. Adv. Cancer Res.; 2014. Gamma-Glutamyl transpeptidase; pp. 103–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunay Y., Altaner S., Ekmen N. The Role of e-NOS in Chronic Cholestasis-Induced Liver and Renal Injury in Rats: the Effect of N-Acetyl Cysteine. Gastroenterol. Res. Pract. 2014;2014:1–8. doi: 10.1155/2014/564949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waisbourd-Zinman O., Koh H., Tsai S., Lavrut P.M., Dang C., Zhao X., Pack M., Cave J., Hawes M., Koo K.A., Porter J.R., Wells R.G. The toxin biliatresone causes mouse extrahepatic cholangiocyte damage and fibrosis through decreased glutathione and SOX17. Hepatology. 2016;64:880–893. doi: 10.1002/hep.28599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao X., Lorent K., Wilkins B.J., Marchione D.M., Gillespie K., Waisbourd-Zinman O., So J., Koo K.A., Shin D., Porter J.R., Wells R.G., Blair I., Pack M. Glutathione antioxidant pathway activity and reserve determine toxicity and specificity of the biliary toxin biliatresone in zebrafish. Hepatology. 2016;64:894–907. doi: 10.1002/hep.28603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahola T., Fellman V., Laaksonen R., Laitila J., Lapatto R., Neuvonen P.J., Raivio K.O. Pharmacokinetics of intravenous N-acetylcysteine in pre-term new-born infants. Eur. J. Clin. Pharmacol. 1999;55:645–650. doi: 10.1007/s002280050687. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues F., Kallas M., Nash R., Cheeseman P., Antiga L.D., Rela M., Heaton N.D. Neonatal hemochromatosis — medical treatment vs . Transplantation . The King ’ s Experience. 2005;11:1417–1424. doi: 10.1002/lt.20497. [DOI] [PubMed] [Google Scholar]
- 27.Mager D.R., Marcon M., Wales P., Pencharz P.B. Use of N-acetyl cysteine for the treatment of parenteral nutrition-induced liver disease in children receiving home parenteral nutrition. J. Pediatr. Gastroenterol. Nutr. 2008;46:220–223. doi: 10.1097/MPG.0b013e3180653ce6. [DOI] [PubMed] [Google Scholar]
- 28.Soghier L.M., Brion L.P. Cysteine, cystine or N-acetylcysteine supplementation in parenterally fed neonates. Cochrane Database Syst. Rev. 2006:CD004869. doi: 10.1002/14651858.CD004869.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flynn D.M., Mohan N., McKiernan P., Beath S., Buckels J., Mayer D., Kelly D.A. Progress in treatment and outcome for children with neonatal haemochromatosis. Arch. Dis. Child. Fetal Neonatal Ed. 2003;88:F124–F127. doi: 10.1136/fn.88.2.F124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahola T., Lapatto R., Raivio K.O., Selander B., Stigson L., Jonsson B., Jonsbo F., Esberg G., Stövring S., Kjartansson S., Stiris T., Lossius K., Virkola K., Fellman V. N-acetylcysteine does not prevent bronchopulmonary dysplasia in immature infants: a randomized controlled trial. J. Pediatr. 2003;143:713–719. doi: 10.1067/S0022-3476(03)00419-0. [DOI] [PubMed] [Google Scholar]
- 31.Mahmoud K.M., Ammar A.S. Effect of N-acetylcysteine on cardiac injury and oxidative stress after abdominal aortic aneurysm repair: a randomized controlled trial. Acta Anaesthesiol. Scand. 2011;55:1015–1021. doi: 10.1111/j.1399-6576.2011.02492.x. [DOI] [PubMed] [Google Scholar]
- 32.E.-H.I., S.L.-M., C.M., P.M., B.D., D.P., C.R., P.P., P.L.P. Effect of intravenous N-acetylcysteine on outcomes after coronary artery bypass surgery: a randomized, double-blind, placebo-controlled clinical trial. J. Thorac. Cardiovasc. Surg. 2007;133:7–12. doi: 10.1016/j.jtcvs.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 33.Shneider B.L., Magee J.C., Karpen S.J., Rand E.B., Narkewicz M.R., Bass L.M., Schwarz K., Whitington P.F., Bezerra J.A., Kerkar N., Haber B., Rosenthal P., Turmelle Y.P., Molleston J.P., Murray K.F., Ng V.L., Wang K.S., Romero R., Squires R.H., Arnon R., Sherker A.H., Moore J., Ye W., Sokol R.J., Alonso E., Kaurs E., Kelly S., Bove K., Heubi J., Miethke A., Tiao G., Denlinger J., Ferris A., Feldman A., Mack C., Suchy F., Sundaram S., Van Hove J., Hite M., Kantor S., Miller T., Smith J., VanWinkle B., Loomes K., Lin H., Piccoli D., Russo P., Spinner N., Brown L., Elgert E., Erlichman J., Alissa F., Lindblad D., Mazariegos G., Ortiz-Aguayo R., Perlmutter D., Sindhi R., Venkat V., Vockley J., Bukauskas K., Kufen A., Schulte M., Bull L., Fleck S., Langlois C., Teckman J., Kociela V., Postma S., Harris K., Bozic M., Subbarao G., Byam B., Klipsch A., Sawyers C., Horslen S., Hsu E., Cooper K., Young M., Kamath B., DeAngelis M., O'Connor C., VanRoestel K., Parmar A., Quammie C., Hung K., Guthery S., Jensen K., Rutherford A., Kerker N., Michail S., Thomas D., Goodhue C., Gupta N., Vos M., de la Cruz-Tracey L., Hankerson-Dyson D., Tory R., Turner-Green T., Wellons A., Brandt M., Finegold M., Harpavat S., Hertel P., Leung D., Liwanag L., Thompson R., Brown S., Doo E., Hoofnagle J., Hall S., Torrance R., Brown J., Liwanag L., Kafka K., Merion R., Spino C. Total serum bilirubin within 3 Months of hepatoportoenterostomy predicts short-term outcomes in biliary atresia. J. Pediatr. 2016;170 doi: 10.1016/j.jpeds.2015.11.058. 211–217.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanjiv Harpavat V., Heubi James E., Karpen Saul J., Lee Ng B.L., David Reginald Setchell Kenneth, Shneider S., Alonso Estella M., Bezerra Jorge A., Guthery J.L., Loomes Kathleen M., Magee John C., Pappas Molleston P.R., Murray Karen F., S R.J., the C.L.D. (ChiLDReN), Averell N., Sherker H., Squires Robert H., Wang Kasper S. Prognostic value of serum bile acids after Kasai portoenterostomy in biliary atresia. Hepatology. 2018;68:85A–86A. doi: 10.1002/hep.32800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venkat V.L., Shneider B.L., Magee J.C., Turmelle Y., Arnon R., Bezerra J.A., Hertel P.M., Karpen S.J., Kerkar N., Loomes K.M., Molleston J., Murray K.F., Ng V.L., Raghunathan T., Rosenthal P., Schwartz K., Sherker A.H., Sokol R.J., Teckman J., Wang K., Whitington P.F., Heubi J.E. Total serum bilirubin predicts fat-soluble vitamin deficiency better than serum bile acids in infants with biliary atresia. J. Pediatr. Gastroenterol. Nutr. 2014;59:702–707. doi: 10.1097/MPG.0000000000000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.