Abstract
Purpose
To investigate whether dexmedetomidine (Dex) can reduce the production of inflammatory factor IL-1β by inhibiting the activation of NLRP3 inflammasome in hippocampal microglia, thereby alleviating the inflammatory response of the central nervous system induced by surgical injury.
Methods
Exploratory laparotomy was used in experimental models in this study. Totally 48 Sprague Dawley male rats were randomly divided into 4 groups (n = 12 for each), respectively sham control (group A), laparotomy only (group B); and Dex treatment with different doses of 5 μg/kg (group D1) or 10 μg/kg (group D2). Rats in groups D1 and D2 were intraperitoneally injected with corresponding doses of Dex every 6 h. The rats were sacrificed 12 h after operation; the hippocampus tissues were isolated, and frozen sections were made. The microglia activation was estimated by immunohistochemistry. The protein expression of NLRP3, caspase-1, ASC and IL-1β were detected by immunoblotting. All data were presented as mean ± standard deviation, and independent sample t test was used to analyze the statistical difference between groups.
Results
The activated microglia in the hippocampus of the rats significantly increased after laparotomy (group B vs. sham control, p < 0.01). After Dex treatment, the number was decreased in a dose-dependent way (group D1 vs. D2, p < 0.05), however the activated microglia in both groups were still higher than that of sham controls (both p < 0.05). Further Western blot analysis showed that the protein expression levels of NLRP3, caspase-1, ASC and downstream cytokine IL-1β in the hippocampus from the laparotomy group were significantly higher than those of the sham control group (all p < 0.01). The elevated expression of these proteins was relieved after Dex treatment, also in a dose-dependent way (D2 vs. D1 group, p < 0.05).
Conclusion
Dex can inhibit the activation of microglia and NLRP3 inflammasome in the hippocampus of rats after operation, and the synthesis and secretion of IL-1β are also reduced in a dose-dependent manner by using Dex. Hence, Dex can alleviate inflammation activation on the central nervous system induced by surgical injury.
Keywords: Dexmedetomidine, Hippocampal microglia, Inflammasome, IL-1β
Introduction
Postoperative cognitive dysfunction (POCD) is a common surgical complication that prolongs hospital stay and increases hospitalization costs. In recent years, many literature have reported that inflammation of central nervous system (CNS) is one of the main causes of POCD.1, 2 IL-1β is one of the important inflammatory factors that cause inflammation of the CNS and ultimately lead to POCD.3, 4 Microglia acts as an immune cell of the CNS, and its activation plays an important role in the occurrence and development of CNS inflammation.5, 6, 7 In addition, surgical injury can induce IL-1 secretion in the CNS, especially in the hippocampus, and result in an inflammatory response.8, 9 Recently, it has been found that activation of NLRP3 is a key pathway for the synthesis and secretion of IL-1β.10 Many studies have found that using Dex can prevent the occurrence of POCD in elderly patients by inhibiting inflammatory factors such as IL-1β, but their molecular mechanisms are still unclear.11, 12 Therefore, this paper intends to establish a surgical injury rat model with laparotomy, and to verify whether Dex can reduce the synthesis and secretion of inflammatory mediators and reduce the CNS inflammatory response by inhibiting the activation of NLRP3 in hippocampal microglia, so as to provide references for Dex administration in preventing POCD.
Methods
Animals and main reagents
Male old rats (12–18 months old) were obtained from the Experimental Animal Center of Daping Hospital of Army Medical University. Dex was purchased from Jiangsu Xinchen Pharmaceutical Company (Jiangsu, China) and 0.3% sodium pentobarbital was purchased from Sigma Company (American). Tissue lysate, BCA protein quantification kit was purchased from Biyuntian Company (China). Antibodies against Iba1 were purchased from Abcam Company, NLRP3 from ZENBIO Company, ASC from Bioss Company, caspase-1 from proteintech Company and IL-1β from Abcam Company.
Establishment of surgical injury models and extraction of hippocampus
Forty-eight rats were randomly divided into 4 groups (n = 12), including group A (sham control), group B (laparotomy only), group D1 (performing laparotomy and intra-abdominal injection of 5 μg/kg Dex) and group D2 (performing laparotomy and intra-abdominal injection of 10 μg/kg Dex). Laparotomy was performed, and the surgical process was as follows. After successful anesthesia with intraperitoneal injection of 0.3% pentobarbital sodium, rats in the group D1 and group D2 were intraperitoneally injected with 5 μg/kg Dex and 10 μg/kg Dex, respectively. Then laparotomy was performed. The abdominal hair was soaked with physiological saline, and the skin was prepared in the operation area and disinfected. Using the scalpel, the skin and subcutaneous tissue were incised along the middle of abdomen with 3 cm-incision, and the muscle was bluntly separated by forceps to reveal the peritoneum, then the peritoneum was open, and the liver, spleen, stomach, small intestine, large intestine and kidney were thoroughly explorated with mosquito forceps. The surgical exploration was performed every 10 min with each exploration for 1 min. The operation lasted for 2 h. At the end of the operation, the wound was sutured according to the anatomical level with 4-0 sterile suture. After suturing, the wound was wrapped with a sterile dressing. Rats in the groups D1 and D2 were injected with their respective doses of Dex every 6 h. Sham group rats were anesthetized with intraperitoneal injection of 0.3% pentobarbital sodium without further operation procedure.
Rats were anesthetized with 0.3% pentobarbital sodium at 12 h after surgery, and the transverse cavity was opened to expose the chest cavity. The right atrial appendage of the mouse was cut open carefully with scissors, and perfused with 50 mL PBS solution for three times through the left ventricle. Finally, the rats were sacrificed by further exsanguination through the abdominal aorta. After decapitation quickly, the rats skull was cut open along the sagittal suture, the brain tissues were removed carefully, cerebellum was separated along the midline, and then the white matter was carefully peeled off with a curved forceps. The hippocampus tissue was then taken out and divided into two parts for preparing frozen sections and extracting proteins, respectively. This protocol has been approved by Animal Ethics Committee of Army Medical University.
Detection of microglia activity by immunohistochemistry
Hippocampal tissue was prepared and cut into 10 μm-thick slices; one slice in every 5 contiguous slices was chosen to undergo the immunostaining. The frozen sections of the hippocampus tissue were rinsed in 0.01 mol/L PBS, incubated in 3% hydrogen peroxide solution (H2O2) for 20 min at room temperature, and re-rinsed with PBS 0.3% Triton X-100 at 37°C for 30 min, then the sections were blocked using 3% fetal calf serum (BSA) for 30 min at room temperature. The specific antibody targeted at microglia was added: rabbit Ib1 (1:1 000) was incubated overnight at 4°C. After rinsing with PBS, biotinylated anti-rabbit IgG (1:200) was added and incubated for another 2 h at room temperature. After rinsing with PBS, the samples were incubated in streptavidin-biotin complex (SABC) for 1 h at 37°C. After PBS rinsing, DAB developed and was naturally dried. Finally the section was sealed with a neutral resin.
After immunostaining, all slices were scanned and pictured under light microscopy (×40). To calculate the number of iba1-labelled microglia of slices, 5 non-overlapping hippocampal CA1 fields in each slice were randomly selected and analyzed. The number of microglias in 5 vision fields were added to calculate the total number of microglias for each slice.
Detection of protein expression of inflammasome and inflammatory factors in hippocampus by Western blot analysis
The other hippocampal tissue samples were weighed and then total protein was extracted by BCA kit from Biyuntian Company (China). The hippocampal tissue samples were put into the lysates buffer (BCA, Biyuntian company, China), homogenized on ice for 30 min, centrifuged, and then the supernatant was harvested. After protein quantification, the lysates were mixed with SDS sample buffer. The proteins were electrophoresed in 12% SDS-PAGE gel and then transferred to polyvinylidene fluoride (PVDF) membranes to block with 5% skim milk for 1 h at room temperature. Membranes were incubated with the following primary antibodies against inflammasome complex (NLRP3, ASC and caspase-1, 1:500) and inflammatory factors (IL-1β) with antibody concentration of 1:1000 were added and incubated overnight. After that, we washed the membranes with 0.1% TBS-tween20 10 mins for 3 times and incubated the membranes with horseradish peroxidase (HRP) labeled corresponding secondary antibody (1:5000) for 1.5 h at room temperature. The OD density values of bands were analyzed using Quantity One Software (Image J image analysis software).
Statistical analysis
All experimental data were presented as mean ± standard deviation. The independent sample T test was used to compare the differences between groups. The statistical analysis was performed using GraphPad Prism 7.04. p < 0.05 was considered to be statistically significant.
Results
Effect of Dex on the activation of rat hippocampal microglia
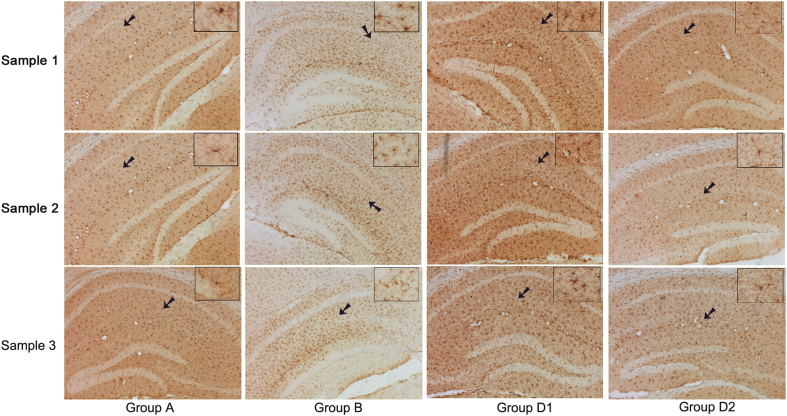
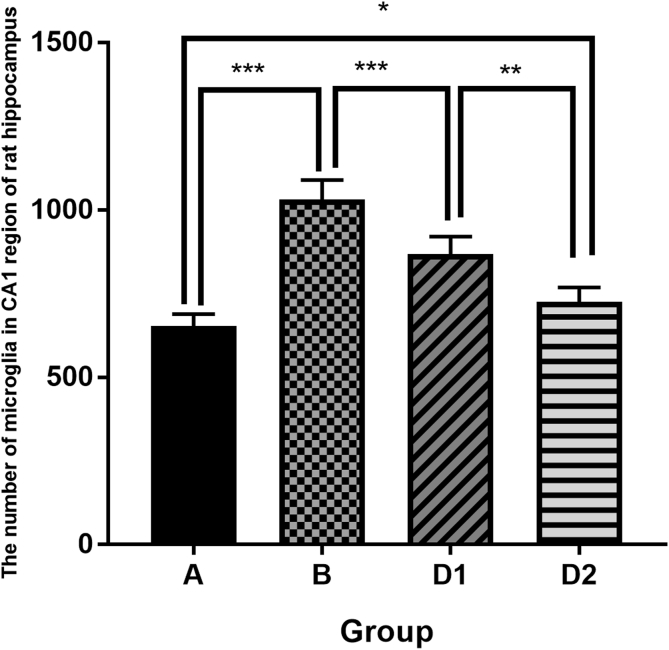
The immunohistochemistry in hippocampus were observed under microscopy and showed that activated microglia (iba1 positive cell) was short-roded, with several branches protruding from the surface. The surface of the protrusion was rough, with caltrop spine and few branches. Its nucleus was small and its shape was irregular, and it was kidney-shaped, elliptical or triangular with many nuclear chromatins (Fig. 1). Further analysis about the number of microglias in CA1 region of rat hippocampus showed that the activated hippocampal microglias in group B (laparotomy only) significantly increased than that in sham control group (1032 ± 23.63 vs. 653.2 ± 14.56, p < 0.01). The activated microglias decreased after Dex treatment (D1 vs. B: 868.7 ± 20.97 vs. 1032 ± 23.63, p < 0.01), and the decrease aggravated with an increasing dose of Dex (D2 vs. D1: 725 ± 17.59 vs. 868.7 ± 20.97, p < 0.01, Fig. 2). It is suggested that surgical injury may stimulate microglia activation in the hippocampus, and Dex could inhibit the activation of microglia in the hippocampus after surgical injury.
Fig. 1.
The activated microglia (×200) of hippocampus in each group was observed under microscope. Group A: sham control group; group B: laparotomy only group, group D1: laparotomy with intraperitoneal injection of 5 μg/kg Dex; group D2: laparotomy with intraperitoneal injection of 10 μg/kg Dex. The brown marker shows iba1 positive microglia cells, and the arrow shows the image after magnifying for 10 times.
Fig. 2.
The number of microglias in CA1 region of rat hippocampus shows that group A < group B (p < 0.01), group D1 < group B (p < 0.01), and group D2 < group D1 (p < 0.01).
Effect of Dex on the expression of inflammasome complex and IL-1β in hippocampal microglia
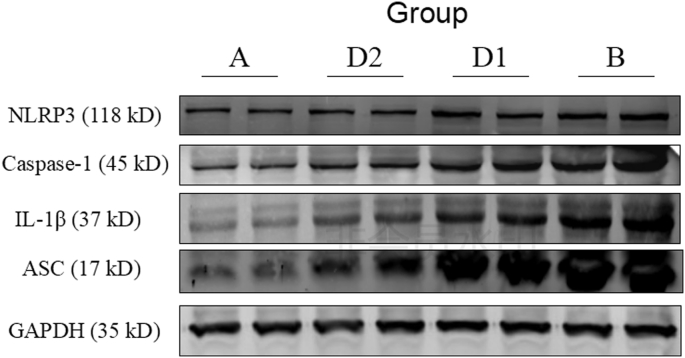
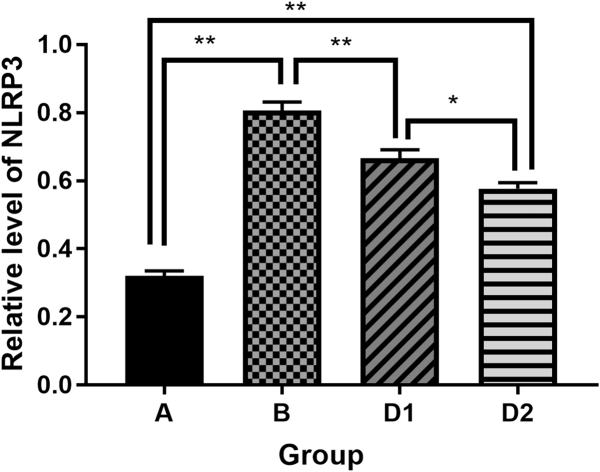
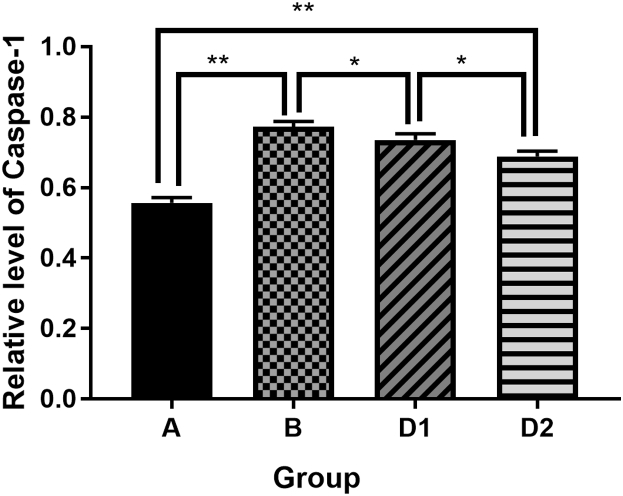
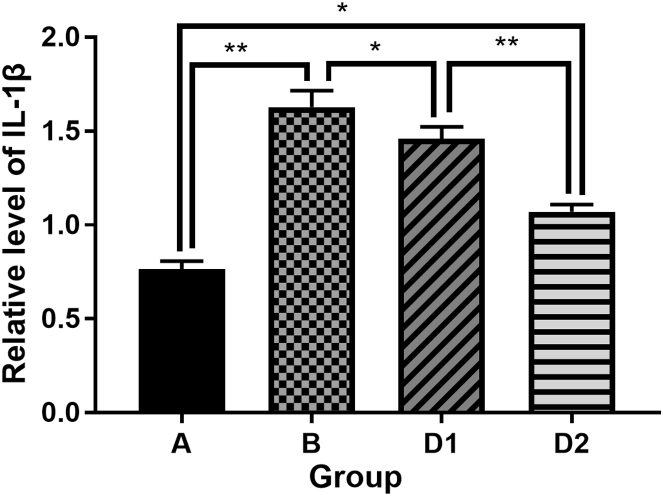
Compared with the sham control group, the expression of inflammasome complex including NLRP3, ASC, caspase-1 and the downstream cytokine IL-1β in hippocampus were significantly increased after laparotomy (p < 0.01). After treatment with Dex, the expression of the above protein molecules in the hippocampus were decreased (D1 vs. B, p < 0.05), and the group D2 were even lower than that of the group D1 (D2 vs. D1, p < 0.05). It is suggested that Dex could depress the high expression of NLRP3, ASC, Caspase-1 and IL-1β in hippocampus from rats which underwent laparotomy, and the depression of Dex was dose dependent (Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7).
Fig. 3.
NLRP3 inflammasome complex and IL-1β were detected by Western blot. Group A: sham control group; group B: laparotomy group, group D1: laparotomy with intraperitoneal injection of 5 μg/kg Dex; group D2: laparotomy with intraperitoneal injection of 10 μg/kg Dex.
Fig. 4.
Comparison of NLRP3 protein expression in each group. Group A: sham control group; group B: laparotomy group, group D1: laparotomy with intraperitoneal injection of 5 μg/kg Dex; group D2: laparotomy with intraperitoneal injection of 10 μg/kg Dex.
Fig. 5.
Comparison of ASC protein expression in each group. Group A: sham control group; group B: laparotomy group, group D1: laparotomy with intraperitoneal injection of 5 μg/kg Dex; group D2: laparotomy with intraperitoneal injection of 10 μg/kg Dex.
Fig. 6.
Comparison of Caspase-1 protein expression levels in each group. Group A: sham control group; group B: laparotomy group, group D1: laparotomy with intraperitoneal injection of 5 μg/kg Dex; group D2: laparotomy with intraperitoneal injection of 10 μg/kg Dex.
Fig. 7.
Comparison of IL-1β protein expression levels in each group. Group A: sham control group; group B: laparotomy group, group D1: laparotomy with intraperitoneal injection of 5 μg/kg Dex; group D2: laparotomy with intraperitoneal injection of 10 μg/kg Dex.
Discussion
The clinical manifestations of postoperative cognitive dysfunction (POCD) in elderly patients include personality changes, weak social skills, cognitive decline, memory impairment, decreased attention and diminished intelligence etc., which last for a long time, causing serious adverse effects on postoperative recovery. However, there are few measures for preventing POCD in clinical practice. In recent years, many literature have reported that CNS inflammation is one of the major causes of POCD.1, 2 Surgical injury can promote the activation of immune cells in the CNS, increase the secretion of inflammatory factors such as IL-1β, thus causing CNS inflammation finally. CNS inflammation induced by IL-1β can destroy neuronal structure, resulting in abnormal neuronal function and eventually POCD. However, the mechanism is still unclear.3, 4 The NLRP3 inflammasome consists of the sensor molecule NLRP3, the adaptor protein ASC, and pro-caspase-1. The NLRP3 protein comprises a pyrin domain (PYD), and the ASC protein harbors PYD and CARD domains. Upon activation, the NLRP3 protein interacts with ASC through PYD, and the CARD domain of ASC recruits the CARD domain of pro-caspase-1 to form NLRP3 inflammasome, which then synthesizes IL-1β, and cleaves the cytokine precursors pro-IL-1β into mature IL-1β.11. Generally, the triggers of NLRP3 activation are mainly tissue fragments after cell disruption, but whether surgical injury could stimulate NLRP3 activation in CNS remains unclear. In addition, since hippocampus takes a major role in learning and memory, whether hippocampal microglia involves in POCD is a pending question.
Additionally, as a selective α2-adrenergic receptor agonist with molecular weight of 200.28 Da, Dex is a commonly used sedative in clinical practice which can reach the brain tissue through the blood-brain barrier. It is reported that Dex can prevent the occurrence of POCD, but the mechanism is still unclear. Literature reported that Dex can inhibit the release of inflammatory factors such as IL-1β.11, 12, 13 Therefore, we speculate that the inhibition role of Dex toward IL-1β may be related to the inhibition of NLRP3 inflammasome activation.
Li et al.14 found that rats which received laparotomy presented prolonger escape latency during morris water maze test. Meanwhile, their space exploration and number of crossing platform decreased significantly after laparotomy. These results suggested surgical injury, such as laparotomy may induce the cognitive dysfunction. Therefore, we used the rat laparotomy model to test inflammatory mechanism of POCD in this study. Hu et al.15 reported that an intraperitoneal injection of Dex 5 μg/kg and 10 μg/kg in the rat sepsis model can inhibit the release of inflammatory factors in the blood of rats. Therefore, the same dose of Dex had been used in this study to verify the effect of Dex on activation of hippocampal microglia after rat laparotomy operation injury, and the NLRP3 inflammasome activation and production of IL-1β from hippocampal microglia were also determined subsequently.
CNS inflammation is one of the main causes of POCD, and over-activation of microglia might be an important link leading to CNS inflammation.16 Several studies have shown that microglia hyper-activation can be stained with immunohistochemistry and labeled with iba1.17 In this experiment, microscopic observation revealed that the number of iba1-labeled activated microglia in laparotomy group was remarkably increased than that of the sham control group. This suggested that surgical injury can activate a large number of microglia in hippocampus. Likewise, administration of Dex can significantly inhibit activation of hippocampal microglia in a dose-dependent way (p < 0.01).
The release of IL-1β by activated microglia can induce CNS inflammation, and leads to affect neuronal function. Among microglia cell, when the receptor protein of NLRP3 is stimulated by its stimuli, caspase-1 is finally activated by the interaction between PYD and CARD, inducing self-cleavage and activation. Activated caspase-1 can promote the maturation of cellular interleukins and other cytokines. Mario Cibelli et al. found that surgical anesthesia could increase the production of IL-1β, reach its peak at 6 h, and then last for more than 12 h after surgery.18 Consequently, these extensive cytokines in plasma facilitate a wide range of inflammatory processes and lead to the destruction of the structural integrity of the blood-brain barrier.19 Inevitably, microglia in brain was activated and released cytokines, including IL-1β after operation.20, 21
Therefore, the POCD rat model was selected in this experiment. The hippocampus specimens were obtained 12 h after operation. The expressions of NLRP3, Caspase-1, ASC and IL-1β in hippocampus tissue were detected by Western blot. The results showed that IL-1β in the sham group A was less than that in the laparotomy group B (p < 0.01), suggesting that there was inflammatory reaction in hippocampus after POCD in rats. Meanwhile, the expression of IL-1β protein was decreased (p < 0.05) after administration of Dex, suggesting that Dex can alleviate the inflammatory response in hippocampus caused by surgical injury. These alleviation effects are proportional to the dose of Dex.
Furthermore, in this experiment, the protein expression of inflammasome complex related components NLRP3, caspase-1 and ASC protein in each group were statistically analyzed. The results were consistent with IL-1β, suggesting that anti-inflammatory effect of Dex may be through inhibition of NLRP3 activation. Dex can reduce ASC protein recruitment, decrease activated Caspase-1, diminish mature IL-1β, and ultimately inhibit the synthesis and release of IL-1β. Therefore, we speculate that Dex can inhibit the activation of NLRP3 in the hippocampal microglia of rats after surgery and reduce the synthesis and secretion of IL-1β in the hippocampus brain tissues in a dose-dependent manner, thereby alleviating the inflammatory activation effect of surgical injury on the CNS.
This experiment only detected protein expression of IL-1β and NLRP3 complex, and activated microglia was determined by performing immunohistochemistry, which may not clarify completely the detailed crosstalk mechanism between over-activated inflammation in CNS and POCD. Further studies on how Dex inhibits the activation of NLRP3 to exert anti-inflammatory effects, and the detailed molecular signal mechanism between inflammation and POCD are needed.
Funding
This work was supported by The National Natural Science Youth Fund of China (No. 81701116).
Ethical statement
This protocol has been approved by Animal Ethics Committee of Army Medical University.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Vacas S., Degos V., Feng X. The neuroinflammatory response of postoperative cognitive decline. Br Med Bull. 2013;106:161–178. doi: 10.1093/bmb/ldt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosczyk H.A., Sparkman N.L., Johnson R.W. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol. 2008;43:840–846. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrientos R.M., Hein A.M., Frank M.G. Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J Neurosci. 2012;32:14641–14648. doi: 10.1523/JNEUROSCI.2173-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraham J., Johnson R.W. Central inhibition of interleukin 1beta ameliorates sickness behavior in aged mice. Brain Behav Immun. 2009;23:396. doi: 10.1016/j.bbi.2008.12.008. 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casal C., Serratosa J., Tusell J.M. Relationship between beta-AP peptide aggregation and microglial activation. Brain Res. 2002;928:76–84. doi: 10.1016/s0006-8993(01)03362-5. [DOI] [PubMed] [Google Scholar]
- 6.Benveniste E.N. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med (Berl) 1997;75:165–173. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- 7.Hu Z., Ou Y., Duan K. Inflammation: a bridge between post operative cognitive dysfunction and Alzheimer's disease. Med Hypotheses. 2010;74:722–724. doi: 10.1016/j.mehy.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 8.Xiong Y., Mahmooda A., Choopp M. Current understanding of neuroinflammation after traumatic brain injury and cell-based therapeutic opportunities. Chin J Traumatol. 2018;21:137–151. doi: 10.1016/j.cjtee.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao X.Z., Ma H., Wang J.K. Postoperative cognitive deficits and neuroinflammation in the hippocampus triggered by surgical trauma are exacerbated in aged rats. Prog Neuro-Psychopharmacol Biol Psychiatry. 2010;34:1426–1432. doi: 10.1016/j.pnpbp.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 10.PY B.F., Kim M.S., Vakifahmetoglu-Norberg H., Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell. 2013;49:331–338. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Chen W., Liu B., Zhang F. The effects of dexmedetomidine on post-operative cognitive dysfunction and inflammatory factors in senile patients. Int J Clin Exp Med. 2015;8:4601–4605. [PMC free article] [PubMed] [Google Scholar]
- 12.Ding L., Zhang H., Mi W. Effects of dexmedetomidine on recovery period of anesthesia and postoperative cognitive function after robot-assisted laparoscopicradical prostatectomy in the elderly people. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2015;40:129–135. doi: 10.11817/j.issn.1672-7347.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Tasdogan M., Memis D., Sut N. Results of a pilot study on the effects of propofol and dexmedetomidine on inflammatory responses and intraabdominal pressure in severe sepsis. J Clin Anesth. 2009;21:394–400. doi: 10.1016/j.jclinane.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Li W., Chai Q., Zhang H. High doses of minocycline may induce delayed activation of microglia in aged rats and thus cannot prevent postoperative cognitive dysfunction. J Int Med Res. 2018;46:1404–1413. doi: 10.1177/0300060517754032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu H., Shi D., Hu C. Dexmedetomidine mitigates CLP-stimulated acute lung injury via restraining the RAGE pathway. Am J Transl Res. 2017;9:5245–5258. [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H.L., Ma R.H., Fang H. Impaired spatial learning memory after isoflurane anesthesia or appendectomy in aged mice is associated with microglia activation. J Cell Death. 2015;8:9–19. doi: 10.4137/JCD.S30596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajan W.D., Wojtas B., Gielniewski B. Dissecting functional phenotypes of microglia and macrophages in the rat brain after transient cerebral ischemia. Glia. 2018;67:232–245. doi: 10.1002/glia.23536. [DOI] [PubMed] [Google Scholar]
- 18.Cibelli M., Fidalgo A.R. Role of interleukin-1β in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terrando N., Eriksson L.I., Ryu J.K. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986–995. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J., Tan H., Jiang W. Amantadine alleviates postoperative cognitive dysfunction possibly by increasing glial cell line-derived neurotrophic factor in rats. Anesthesiology. 2014;121:773–785. doi: 10.1097/ALN.0000000000000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J., Jiang W., Zuo Z. Pyrrolidine dithiocarbamate attenuates surgery-induced neuroinflammation and cognitive dysfunction possibly via inhibition of nuclear factor κB. Neuroscience. 2014;261:1–10. doi: 10.1016/j.neuroscience.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]