Abstract
Objectives
The Joint United Nations Programme on HIV/AIDS (UNAIDS) targets aim to reduce new HIV infections below 500,000 per year by 2020. Despite targeted prevention programmes, total new infections remained in 2016 and 2017 at 1,800,000 cases. We have aimed to analyse data from 2017 and to compare HIV incidence, AIDS-related deaths and provision of antiretroviral therapy (ART) to adults, pregnant women and children living with HIV in lower- and higher-prevalence countries. Vertical or mother-to-child transmission (MTCT) and early infant diagnosis (EID) rates were also investigated.
Methods
UNAIDSinfo data use the Spectrum model to represent country-level HIV data. Countries with epidemics over 40,000 HIV cases were separated into higher prevalence (≥4.5%) and lower prevalence (<4.5%). Least squares linear regression, weighted by epidemic size and controlled for gross domestic product/capita, was used to compare HIV prevalence with estimated ART coverage in adults (≥15 years), children (0–14 years), pregnant women, and EID rates and MTCT rates. Data were then compared between higher- and lower-prevalence groups, including numbers of new HIV infections and AIDS-related deaths.
Results
Data were available for 56 countries. Twelve higher-prevalence countries accounted for 16.7 million and 44 lower-prevalence ones for 15.1 million people living with HIV, altogether making up 87.5% of the global estimate. Lower-prevalence countries had less ART coverage for adults, pregnant women and children, lower EID rates and higher AIDS-related death levels. There were more new HIV infections in adults and children in lower- than higher-prevalence countries.
Conclusions
Most new HIV infections, MTCTs and AIDS-related deaths occurred in countries with an HIV prevalence rate below 4.5%. Many of these countries are not targeted by access programmes, such as the President’ Emergency Plan for AIDS Relief. More intensive programmes of diagnosis and treatment are needed in these countries in the effort to reduce global new HIV infections below 500,000 per year by 2020.
Keywords: new HIV infections, prevalence, antiretroviral therapy, early infant diagnosis, mother-to-child transmission
Introduction
The Joint United Nations Programme on HIV/AIDS (UNAIDS) and partners launched in 2014 the 90–90–90 targets. The aim was to diagnose 90% of all HIV-positive individuals, provide antiretroviral therapy (ART) for 90% of those diagnosed and achieve viral suppression for 90% of those treated by 2020 [1].
In 2016, key prevention targets were added to reduce HIV incidence and HIV/AIDS-related deaths by 2020, such as reductions in the number of new infections to below 500,000 per year and below 100,000 for adolescent girls and young women; to have 3 million people treated with HIV pre-exposure prophylaxis (PrEP) worldwide; to provide 90% of key populations with access to combined HIV prevention; and to reduce total HIV/AIDS-related deaths to below 500,000 per year [1,2].
In order to reach these targets, UNAIDS echoed the 2011 Global Plan to eliminate new childhood infections by 2015 by emphasising the need to focus on ‘treatment as prevention’ and core prevention strategies, such as elimination of vertical or mother-to-child-transmission (MTCT) [3], alongside condoms, PrEP, male circumcision, needle exchange and public health education methods [4]. They also set specific targets for accessing key populations.
Programmes of prevention of mother-to-child transmission (pMTCT) have indeed been credited with contributing to a reduction in childhood HIV incidence, as ART coverage for pMTCT increased from 50% to 77% between 2010 and 2015 [5]. In 2015, World Health Organization (WHO) guidelines for pMTCT settled on option B+, which involves lifelong ART for all pregnant HIV-positive women and all children born to HIV-positive mothers given nevirapine or zidovudine daily from birth to 4–6 weeks. The option B+ success can be measured with early infant diagnosis (EID), without which treatment of the newborn may not take place. For this reason, EID is increasingly emphasised as a means of measuring levels of care in newborns at potential risk of HIV infection [1]. Considered alongside EID, option B+ would be expected to further contribute to decreased HIV incidence as seen between 2010 and 2015.
With renewed emphasis on treatment as prevention and therefore ART coverage and pMTCT, it would be expected that AIDS-related deaths, MTCT and total new HIV infections would continue to fall. Whilst AIDS-related deaths and total new infections have fallen by 34% and 18%, respectively, since 2010, worldwide new infections in 2016 and 2017 have remained constant at 1.8 million cases, 180,000 of whom are children [6–8]. At the current reduction rate of new HIV infection, the 2020 target of fewer than 500,000 new cases per year remains out of reach. This suggests a need for a new approach [9].
Initiatives such as the Global Fund and the United States President's Emergency Plan for AIDS Relief (PEPFAR) have consistently had regional focuses. Similarly, the 2011 Global Plan has worked in 22 priority countries selected on the basis of HIV prevalence in pregnant woman and country–income classifications. Here there was a 60% decrease in new HIV infections in children. As the US aid and the Global Fund budgets are reduced [10], the trend has intensified to focus on higher-prevalence settings, following increased pressure for programme cost-effectiveness. Last year, PEPFAR announced that it would now focus efforts on just 13 priority countries [11]. This resource allocation strategy raises the question of whether there is a difference in treatment levels in lower-prevalence vs higher-prevalence countries and, if so, is this difference reflected in incidences and outcomes for these HIV-positive populations?
In this analysis, we have aimed to investigate whether there was a prevalence bias in ART provision for people living with HIV (PLWHIV) in 2017, looking more specifically at adults, pregnant women and children. We have explored a potential difference in the number of AIDS-related deaths and new HIV infections between lower- and higher-prevalence countries. To gain insight into HIV-positive pregnant women and children outcomes, we have also examined EID and MTCT rates. We have compared estimates amongst 56 higher- and lower-prevalence countries with an epidemic size above 40,000 cases. We have used for the year 2017 the UNAIDSinfo database, which is the most extensive and detailed dataset for these variables.
Methods
The UNAIDSinfo database provides estimates for a wide variety of epidemiological data, including HIV prevalence, epidemic size, ART coverage for adults, children and pregnant women, as well as numbers of new HIV infections and HIV/AIDS-related deaths and MTCT and EID rates [7]. This study has analysed UNAIDSinfo data from within the year 2017.
Countries included in this study had an epidemic size of over 40,000 PLWHIV. Those in the regions of North America, Oceania and Western Europe were excluded due to significant differences in epidemic demographic and national income levels compared with the rest of the world. We have excluded four further countries, Colombia, Thailand, Vietnam and China, due to a lack of information in terms of numbers of new infections.
Countries included in the analysis were categorised as higher or lower prevalence, with ≥4.5% considered as higher or <4.5% as lower. This threshold split the global HIV-positive population into two, such that each group accounted for a similar proportion, with the higher-prevalence group accounting for 52% and the lower-prevalence group accounting for 48%.
Data on MTCT rates, defined as the percentage of children born to HIV-positive mothers who were themselves infected by 12 months of age, were taken from the UNAIDS Spectrum model and were cross-referenced with WHO country reports for countries whenever available. A new child infection was defined as a new HIV diagnosis made before 14 years of age, as the vast majority of these new infections are vertical transmissions and only a small minority are horizontal ones (sexual or blood product related) [12]. We defined pMTCT as the estimated percentage of pregnant women undergoing ART to prevent HIV vertical transmission.
Least squares linear regression, weighted by total adult HIV epidemic size and controlling for gross domestic product per capita, was used to correlate HIV prevalence with estimated rates of EID, MTCT and ART coverage for children, adults and pregnant women. Comparisons were made for lower- and higher-prevalence groups on numbers of new adult and child infections, ART coverage for adults, children and pregnant women, as well as numbers of AIDS-related deaths for adults and children, and rates of MTCT and EID as defined as an HIV test within 2 months of delivery.
Results
A total of 56 countries were included in our analysis. Twelve higher-prevalence countries accounted for 16.7 million and 44 lower-prevalence countries accounted for 15.1 million PLWHIV. These countries made up for 87.5% of the global epidemic size, 87% of global new infections and 89% of global AIDS-related deaths. There were 4.1 new infections per 100 PLWHIV in higher-prevalence countries vs 5.8 per 100 PLWHIV in lower-prevalence countries. Tables 1 and 2 show the countries included in this analysis in decreasing order of the number of new infections. Estimates for new HIV infections, epidemic size, HIV prevalence, MTCT and EID rates, ART coverage and AIDS-related deaths are included.
Table 1.
Total new infections and ART coverage in adults living with HIV by region: (a) higher prevalence and (b) lower prevalence
| a | |||||
|---|---|---|---|---|---|
| Country | Epidemic size | HIV prevalence (%) | Total new infections (n) | % ART coverage for PLWHIV | AIDS-related deaths |
| Eastern and Southern Africa | 16,610,000 | 674,400 | 304,200 | ||
| South Africa | 7,200,000 | 18.80 | 270,000 | 61 | 110,000 |
| Mozambique | 2,100,000 | 12.50 | 130,000 | 54 | 70,000 |
| *Kenya* | 1,500,000 | 4.80 | 53,000 | 75 | 28,000 |
| *Uganda* | 1,300,000 | 5.90 | 50,000 | 72 | 26,000 |
| Zambia | 1,100,000 | 11.50 | 48,000 | 75 | 16,000 |
| *Zimbabwe* | 1,300,000 | 13.30 | 41,000 | 84 | 22,000 |
| *Malawi* | 1,000,000 | 9.60 | 39,000 | 71 | 17,000 |
| *Lesotho* | 320,000 | 23.80 | 15,000 | 74 | 4900 |
| *Botswana* | 380,000 | 22.80 | 14,000 | 84 | 4100 |
| *Namibia* | 200,000 | 12.10 | 7400 | 84 | 2700 |
| *Swaziland* | 210,000 | 27.40 | 7000 | 85 | 3500 |
| Central Africa | 53,000 | 4100 | 1900 | ||
| Equatorial Guinea | 53,000 | 6.50 | 4100 | 38 | 1900 |
| Total | 16,663,000 | — | 678,500 | — | 306,100 |
| Weighted average | — | 14.45 | — | 67 | — |
| b | |||||
|---|---|---|---|---|---|
| Country | Epidemic size | HIV prevalence (%) | Total new infections (n) | % ART coverage for PLWHIV | AIDS-related deaths |
| Western and Central Africa | 5,972,000 | 370,200 | 263,400 | ||
| Nigeria | 3,100,000 | 2.80 | 210,000 | 33 | 150,000 |
| *Côte d'Ivoire* | 500,000 | 2.80 | 30,000 | 46 | 24,000 |
| Cameroon | 510,000 | 3.70 | 28,000 | 49 | 24,000 |
| Ghana | 310,000 | 1.70 | 19,000 | 40 | 16,000 |
| DRC | 390,000 | 0.70 | 15,000 | 55 | 2600 |
| Mali | 130,000 | 1.20 | 9900 | 32 | 6300 |
| Guinea | 120,000 | 1.50 | 8100 | 35 | 5100 |
| Congo | 100,000 | 3.10 | 7900 | 29 | 4900 |
| Central African Republic | 110,000 | 4.0 | 7700 | 32 | 5200 |
| Chad | 110,000 | 1.30 | 5800 | 45 | 3100 |
| Togo | 110,000 | 2.10 | 4900 | 57 | 4700 |
| Burkina Faso | 94,000 | 0.80 | 4300 | 65 | 2900 |
| Benin | 70,000 | 1.0 | 4000 | 55 | 2500 |
| Sierra Leone | 61,000 | 1.40 | 3200 | 39 | 2600 |
| Burundi | 78,000 | 1.10 | 3100 | 77 | 1700 |
| Gabon | 56,000 | 4.20 | 3100 | 59 | 1300 |
| Guinea-Bissau | 40,000 | 3.40 | 2300 | 30 | 1900 |
| Liberia | 40,000 | 1.40 | 2300 | 29 | 2500 |
| Senegal | 43,000 | 0.40 | 1600 | 54 | 2100 |
| Asia and the Pacific | 3,370,000 | 170,690 | 128,700 | ||
| India | 21,000,000 | 0.20 | 88,000 | 56 | 69,000 |
| Indonesia | 630,000 | 0.40 | 49,000 | 14 | 39,000 |
| Pakistan | 150,000 | 0.10 | 20,000 | 8 | 6200 |
| Malaysia | 87,000 | 0.40 | 7800 | 45 | 4400 |
| Papua New Guinea | 48,000 | 0.90 | 3000 | 55 | 1100 |
| Philippines | 68,000 | 0.10 | 1200 | 36 | 1000 |
| Myanmar | 220,000 | 0.70 | 1100 | 66 | 6700 |
| Cambodia | 67,000 | 0.50 | 590 | 87 | 1300 |
| Eastern Europe and Central Asia | 1,292,000 | 119,400 | 10,900 | ||
| Russia | 1,000,000 | 1.20 | 100,000 | 36 | nd |
| Ukraine | 240,000 | 0.90 | 13,000 | 40 | 9000 |
| Uzebekistan | 52,000 | 0.30 | 6400 | 29 | 1900 |
| Latin America | 1,382,000 | 74,600 | 24,100 | ||
| Brazil | 860,000 | 0.60 | 48,000 | 64 | 14,000 |
| Mexico | 230,000 | 0.30 | 15,000 | 62 | 4000 |
| Argentina | 120,000 | 0.40 | 6,500 | 66 | 2000 |
| Peru | 72,000 | 0.30 | 2,800 | 67 | 2100 |
| Guatemala | 46,000 | 0.40 | 2,300 | 39 | 2000 |
| Eastern and Southern Africa | 2,820,000 | 129,400 | 75,100 | ||
| *Tanzania* | 1,500,000 | 4.50 | 65,000 | 66 | 32,000 |
| Angola | 310,000 | 1.90 | 27,000 | 26 | 13,000 |
| Ethiopia | 610,000 | 0.90 | 16,000 | 71 | 15,000 |
| South Sudan | 180,000 | 2.40 | 14,000 | 13 | 12,000 |
| *Rwanda* | 220,000 | 2.70 | 7400 | 83 | 3100 |
| Carribean | 217,000 | 10,000 | 7300 | ||
| *Haiti* | 150,000 | 1.90 | 7600 | 64 | 4700 |
| Dominican Republic | 67,000 | 0.90 | 2400 | 52 | 2600 |
| Middle East and Northern Africa | 111,000 | 9400 | 6100 | ||
| Sudan | 51,000 | 0.20 | 4700 | 15 | 2600 |
| Iran | 60,000 | 0.10 | 4700 | 19 | 3500 |
| Total | 15,110,000 | — | 883,690 | — | 530,000 |
| Weighted average | — | 1.81 | — | 47 | — |
*...*: countries supported by PEPFAR. ART: antiretroviral therapy; PLWHIV: people living with HIV.
Table 2.
Total new child infections, MTCT rates, EID and ART coverage by region: (a) higher prevalence and (b) lower prevalence
| a | ||||||
|---|---|---|---|---|---|---|
| Country | New child infections (n) | MTCT rate (%) | % EID 2017 | % ART coverage for pMTCT | % ART coverage for children | Child AIDS-related deaths |
| Eastern and Southern Africa | 66,050 | 39,590 | ||||
| Mozambique | 18,000 | 15 | 50 | 86 | 51 | 9800 |
| South Africa | 13,000 | 5 | 95 | 95 | 58 | 8600 |
| *Kenya* | 8000 | 12 | 51 | 76 | 82 | 4300 |
| *Uganda* | 7600 | 8 | 48 | 95 | 68 | 3800 |
| *Zambia* | 7300 | 10 | 46 | 92 | 64 | 3400 |
| *Malawi* | 4900 | 9 | 52 | 92 | 63 | 3000 |
| *Zimbabwe* | 4300 | 7 | 65 | 95 | 89 | 4300 |
| *Lesotho* | 890 | 7 | 51 | 90 | 60 | 890 |
| *Swaziland* | 850 | 10 | 81 | 90 | 75 | 500 |
| *Botswana* | 610 | 5 | 50 | 90 | 68 | 500 |
| *Namibia* | 600 | 6 | 95 | 95 | 76 | 500 |
| Central Africa | 540 | 500 | ||||
| Equatorial Guinea | 540 | 23 | nd | 64 | 17 | 500 |
| Total | 66,590 | — | — | — | — | 40,090 |
| Weighted average | — | 8 | 71 | 91 | 64 | — |
| b | ||||||
|---|---|---|---|---|---|---|
| Country | New child infections (n) | MTCT rate (%) | % EID 2017 | % ART coverage for pMTCT | % ART coverage in children | Child AIDS-related deaths |
| Western and Central Africa | 65,800 | 41,460 | ||||
| Nigeria | 36,000 | 23 | 12 | 30 | 26 | 23,000 |
| DRC | 4800 | 21 | 34 | 59 | 58 | 100 |
| Cameroon | 4500 | 15 | 51 | 77 | 25 | 3300 |
| *Côte d'Ivoire* | 3800 | 15 | 40 | 70 | 27 | 3100 |
| Ghana | 3400 | 19 | 30 | 66 | 23 | 2900 |
| Mali | 2000 | 27 | 11 | 31 | 23 | 980 |
| Congo | 1700 | 27 | 3 | 11 | 18 | 1200 |
| Guinea | 1500 | 24 | 11 | 38 | 18 | 720 |
| Chad | 1300 | 17 | 5 | 68 | 18 | 850 |
| Togo | 1200 | 20 | 36 | 66 | 30 | 870 |
| Central African Republic | 1100 | 22 | 23 | 56 | 25 | 700 |
| Burundi | 690 | 14 | 20 | 85 | 38 | 500 |
| Benin | 660 | 14 | 32 | 83 | 27 | 540 |
| Burkina Faso | 660 | 12 | 16 | 92 | 28 | 500 |
| Sierra Leone | 560 | 13 | 7 | 89 | 18 | 500 |
| Guinea-Bissau | 510 | 23 | 36 | 65 | 16 | 500 |
| Gabon | 500 | 17 | 4 | 64 | 50 | 200 |
| Liberia | 500 | 28 | nd | 86 | 18 | 500 |
| Senegal | 500 | 22 | 23 | 53 | 25 | 500 |
| Eastern and Southern Africa | 24,660 | 14,900 | ||||
|---|---|---|---|---|---|---|
| *Tanzania* | 11,000 | 12 | 36 | 85 | 46 | 6000 |
| Angola | 5500 | 26 | 1 | 34 | 14 | 3300 |
| Ethiopia | 5500 | 17 | 38 | 59 | 34 | 3600 |
| South Sudan | 1800 | 20 | 10 | 60 | 9 | 1500 |
| *Rwanda* | 860 | 9 | 85 | 92 | 76 | 500 |
| Asia and the Pacific | 9400 | 6630 | ||||
|---|---|---|---|---|---|---|
| India | 3700 | 16 | 23 | 60 | nd | 2600 |
| Indonesia | 3100 | 26 | 1 | 13 | 25 | 2200 |
| Pakistan | 950 | 31 | 1 | 6 | 13 | 530 |
| Philippines | 200 | 40 | 5 | 11 | 13 | 100 |
| Myanmar | 750 | 13 | 28 | 78 | 91 | 500 |
| Cambodia | 100 | 10 | 64 | 95 | 95 | 100 |
| Malaysia | 100 | 20 | 95 | 95 | 95 | 100 |
| Papua New Guinea | 500 | 29 | 35 | 41 | 40 | 500 |
| Carribean | 1050 | 4000 | ||||
|---|---|---|---|---|---|---|
| *Haiti* | 950 | 15 | 40 | 70 | 50 | 600 |
| Dominican Republic | 100 | 10 | 80 | 95 | 34 | 3,400 |
| Latin America | 1920 | 1280 | ||||
|---|---|---|---|---|---|---|
| Brazil | 720 | 8 | 45 | 85 | 45 | 680 |
| Guatemala | 500 | 42 | 17 | 21 | 42 | 200 |
| Mexico | 500 | 23 | nd | 49 | 69 | 200 |
| Argentina | 100 | 6 | 66 | 90 | 95 | 100 |
| Peru | 100 | 10 | 78 | 84 | 78 | 100 |
| Middle East and Northern Africa | 660 | 600 | ||||
|---|---|---|---|---|---|---|
| Iran | 100 | 20 | 31 | 55 | 53 | 100 |
| Sudan | 560 | 29 | nd | 7 | 19 | 500 |
| Eastern Europe and Central Asia | 500 | 500 | ||||
|---|---|---|---|---|---|---|
| Ukraine | 500 | 17 | 48 | 81 | 54 | 500 |
| Russia | nd | nd | 84 | nd | nd | nd |
| Uzebekistan | nd | nd | 56 | 62 | nd | nd |
| Total | 104,070 | — | — | — | — | 69,370 |
| Weighted average | — | 17 | 30 | 53 | 29 | — |
*...*: countries supported by PEPFAR. ART: antiretroviral therapy; EID: early infant diagnosis; MTCT: mother-to-child transmission; nd: not detected; pMTCT: prevention of mother-to-child transmission.
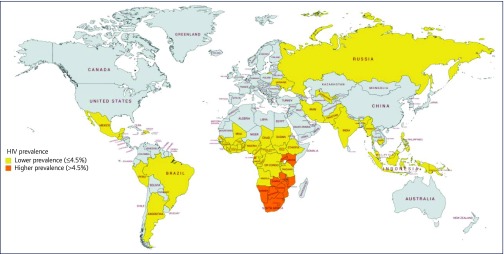
All higher-prevalence countries were located in sub-Saharan Africa, as shown in Figure 1. Table 1a shows that 43% of PLWHIV in higher-prevalence countries lived in South Africa.
Figure 1.
Map of the countries included in this analysis split by HIV prevalence
Within lower-prevalence countries, 63% of the epidemic occurred in Western and Central Africa, 24% in Eastern and Southern Africa, and 9% in Asia and the Pacific, with the remaining 4% made up of the Caribbean, Latin America, the Middle East, Northern Africa, and Eastern Europe and Central Asia combined.
Adult population
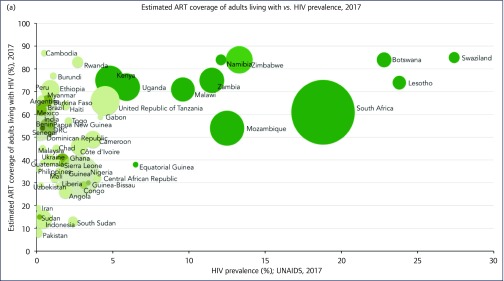
Adult ART coverage was greater in higher-prevalence countries. Weighted average ART coverage was at 67% in higher-prevalence countries vs 47% in lower-prevalence ones. As shown on Figure 2a, as HIV prevalence decreased, so did ART coverage (P = 0.00325). More AIDS-related deaths amongst adults occurred in lower-prevalence countries (n = 530,000) than in higher-prevalence ones (n = 306,100).
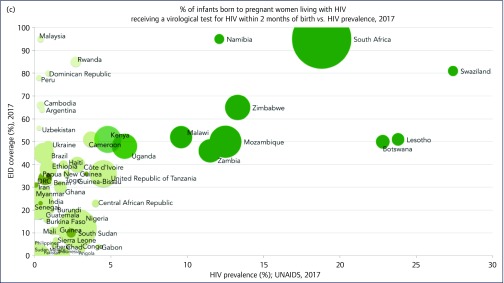
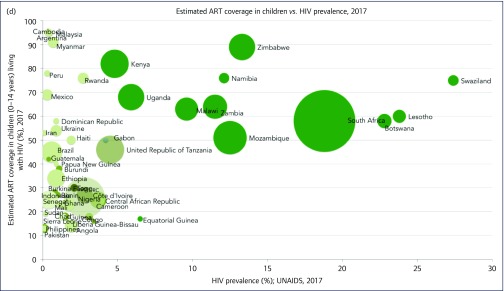
Figure 2.
(a) ART coverage against HIV prevalence weighted by epidemic size. (b) ART coverage in pregnant women against HIV prevalence weighted by epidemic size. (c) Early infant diagnosis coverage (%) against HIV prevalence weighted by epidemic size. (d) ART coverage in children against HIV prevalence weighted by epidemic size. ART: antiretroviral therapy; EID: early infant diagnosis; pMTCT: prevention of mother-to-child transmission; UNAIDS: Joint United Nations Programme on HIV/AIDS
Table 3 shows a summary of HIV data. In the 56 countries analysed, there were a total of 1,562,190 new infections, with 57% of these occurring in the lower-prevalence countries. More specifically, more than 24% of these new infections in lower- prevalence countries occurred in Nigeria (210,000) and 11% (100,000) occurred in Russia.
Table 3.
Summary of HIV data for 2017 showing weighted averages by HIV prevalence, UNAIDS
| Higher prevalence (>4.5%) | Lower prevalence (≤4.5%) | |
|---|---|---|
| No. of countries | 12 | 44 |
| Epidemic size (n) | 16,663,000 | 15,110,000 |
| Prevalence (%) | 14.45 | 1.81 |
| % MTCT | 8 | 17 |
| % EID | 71 | 30 |
| % ART coverage for pMTCT | 91 | 53 |
| % ART coverage for PLHIV | 67 | 47 |
| % ART coverage for children | 64 | 29 |
| New child infections | 66,590 | 104,070 |
| New total infections | 678,500 | 883,690 |
| Child AIDS-related deaths | 40,090 | 69,370 |
| Total AIDS-related deaths | 306,100 | 530,000 |
ART: antiretroviral therapy; EID: early infant diagnosis; MTCT: mother-to-child transmission; PLWHIV: people living with HIV; pMTCT: prevention of mother-to-child transmission; UNAIDS: Joint United Nations Programme on HIV/AIDS.
Pregnant women and children
Vertical transmission transmission
The coverage of pMTCT increased as national HIV prevalence increased (P = 0.028), as shown on Table 3. The pMTCT rate was 53% for pregnant women living in lower-prevalence countries and 91% in higher-prevalence ones. As national HIV prevalence increased, so did national MTCT rates (P = 0.005). Rates of MTCT were 17% in lower-prevalence countries and 8% in higher-prevalence ones.
The majority of higher-prevalence countries had an MTCT rate at 10% or below, with the lowest rates found in Botswana and South Africa. Within the lower-prevalence category, Rwanda, Brazil and Argentina achieved an MTCT rate below 10%. Nearly half of lower-prevalence countries had an MTCT rate of 20% or greater. Nigeria was found to have the highest number of new child infections (36,000) and an MTCT rate at 23%.
Early infant diagnosis
As national HIV prevalence increased, EID rates also increased (P = 0.027). EID was on average 30% vs 71% in the lower- and higher-prevalence countries, respectively. Figure 2c illustrates the poor EID coverage in lower-prevalence countries, particularly Angola, Chad and Sierra Leone at 1%–2%, 5% and 7%, respectively. Rwanda, Peru and Malaysia all showed a higher EID coverage above 75%. Higher-prevalence countries, such as South Africa, Namibia and Swaziland, all achieved an EID coverage above 80%.
Children living with HIV
Figure 2d shows that childhood ART coverage increased with HIV prevalence. Two important anomalies involve Equatorial Guinea in the higher-prevalence category and Rwanda in the lower- prevalence one, with a child ART coverage of 18% and 76%, respectively. ART coverage was higher in higher-prevalence countries (average of 64%) compared with lower-prevalence ones (29%).
There was a total of 170,660 new childhood infections in the 56 countries analysed, with 61% (n = 91,470) in lower- prevalence countries. Furthermore, more childhood AIDS-related deaths occurred in lower-prevalence countries (n = 69,370; 8% of HIV-positive children) compared with higher-prevalence ones (n = 40,090; 4% of HIV-positive children), with death rates twice as high for these children in lower-prevalence countries vs higher-prevalence countries.
Discussion
We have found in the present analysis that 205,190 more new HIV infections occurred in lower-prevalence countries compared with higher-prevalence ones, despite the fact that they accounted for 48% of the global epidemic size and that 63% of AIDS-related deaths took place in lower-prevalence countries. Four key factors may explain these results, such as a lower adult ART and pMTCT coverage and higher MTCT rates, as well as lower EID and childhood ART coverage in lower-prevalence countries. However, different types of distributions of key populations in lower-prevalence countries may also contribute to this higher number of new infections [13]. Lower ART coverage in lower-prevalence countries was seen alongside a greater number of AIDS-related deaths, with almost twice as many in lower-prevalence countries as compared with higher-prevalence countries. Our results highlight an increasing need for effective preventative and treatment programmes in lower-prevalence countries.
There are several strategies used in higher-prevalence settings that could potentially be applied in lower-prevalence ones, including healthcare system decentralisation [14], task shifting, ART distribution by community health workers and mobile technology [15,16], adherence clubs, support networks and adoption of WHO guidelines to test and treat [17,18]. Other ground-breaking advances in ART delivery expansion include home-based, point-of-care testing [19,20], and active recalling and community-based self-testing, especially in groups of men who have sex with men [21,22]. Improved access to testing can also be made possible by lower cost testing kits [23,24]. The international community has galvanised large amounts of funding and grassroots action in higher-prevalence countries, which could be replicated with political will in lower-prevalence countries, which often have unique epidemiological, technical and social challenges in ART delivery [25]. These include lower levels of both community and healthcare professionals’ awareness and understanding of HIV prevention. The absence of dialogue and of HIV testing can lead to increased stigmatising opinions about HIV [26,27] compounded by the difficulty in obtaining monitoring laboratory treatment tools and access to treatment centres. Recent success in Tanzania with the use of drones may suggest one of the ways forward to reach remote areas [28]. Reducing stigma remains crucially important in these lower-prevalence settings [29–31].
Other research exploring the relationship between successful HIV treatment programmes and the national Global Peace Index, Corruption Index and HIV prevalence found that a country with high levels of conflict and of corruption and a lower HIV prevalence was more likely to have a less effective HIV treatment programme [32]. There may be many other complex inter-related factors that explain the correlation between lower HIV prevalence and higher new infection rates.
Factors such as subnational prevalence rates may need further attention [33]. For example, regional prevalence in South Africa ranges from 13.9% to 27%. The Arc Geographic Information System shows that some hotspots have a prevalence of up to over 35%, whilst others have a prevalence of less than 1.6% HIV [34]. Therefore, if we look at lower-prevalence countries with poor national ART coverage, we may find that small hotspot areas, which may include the majority of new infections with high numbers of key populations, have very poor ART coverage, such as in Nigeria [35]. The role of geospatial analysis is increasing, but the level of detail assessed needs to be adjusted to the most relevant one for decision-making in HIV programme planning [36].
Challenges for testing and ART implementation in key populations are well recognised as these often do not disclose their current or former status as at-risk populations, particularly in countries with a high degree of stigmatisation or severe punishment. Furthermore, tracking these individuals and collecting data can be difficult as they may use a variety of services, with testing and treatment in different settings [37]. Lower-prevalence countries may face particular challenges with clusters of ‘hard-to-reach’ high-risk populations, and particular attention should be paid to improve their access to treatment [38].
When considering the populations of mothers and children, we have noted a great disparity in pMTCT between higher-prevalence (91%) and lower-prevalence (49%) countries, with a need for option B+ to be rolled out in the neglected countries. The pMTCT programmes are proving successful in some of the countries with higher HIV prevalence. For example, both Botswana and South Africa are approaching a level below 5% of MTCT (for breastfeeding mothers) set by the WHO as a step towards elimination of mother-to-child transmission (eMTCT). Equatorial Guinea, however, has only 64% pMTCT coverage, with 23% MTCT rates, well above the 8% average with a huge variation in incidence and HIV information across the country [39]. Amongst lower-prevalence countries, Nigeria and Angola have an ART coverage below 35% and an ART coverage of pregnant women at 30% and 34%, respectively. Testing and treatment coverage for children in lower-prevalence countries needs attention, where EID rates are low. Point-of-care testing has not been available for infants and can be costly in areas where test volumes are low. Furthermore, interruption in drug supply and loss-to-follow-up rates can be high despite an aim for lifelong treatment for mothers [40,41]. Wide-scale EID testing is possible as shown in a pilot study in Kenya showing the impact of innovation using text-messaging services [42].
It has to be stressed that not all lower-prevalence countries are performing badly. Ethiopia, the Democratic Republic of Congo and Rwanda all show that improved EID and ART coverage is possible in these settings. Cuba, Belarus and Armenia, not included in the analysis because of small epidemic sizes, have achieved eMTCT [38]. There are clusters of poorly performing countries with ART coverage of less than 40%, EID below 30% and MTCT above 15% in lower-prevalence countries. These should draw on the successes in other low-prevalence countries to improve access to antenatal care, maternal screening, treatment and infant follow-up [38]. Further guidance might come from other successes in paediatric inpatient settings, nutrition centres, immunisation clinics and paediatric outpatient clinics, and triggered testing may have a similar yield to universal testing [43].
There are limitations in our analyses. The UNAIDSinfo database uses the Spectrum model. Given the limited availability of surveillance data across the HIV population, the data presented rely on modelling approaches to fill in the gaps and on modelled understanding of HIV transmission [44,45]. Although the 2017 data were produced by an updated model reflecting improved understanding of MTCT, overestimations and underestimations cannot be excluded [44]. The benefit of using UNAIDSinfo data rather than heterogeneous national reports or peer-reviewed published studies is that data for each country are calculated by the Spectrum model and used the same methodology and are relatively homogenous. Therefore, data for each country both benefit and suffer from the same modelling underestimations and overestimations.
Overall, HIV data remain incomplete, necessitating further estimates, such as for pregnant women with an unknown HIV status or for those not using antenatal services. We have assumed that most new children infections (between the ages 0 and 14) would originate from vertical transmission. Many women breastfeed their children up to the age of 5 years or even longer and may transmit after the 12-month definition, whilst other pregnant women who may have been HIV negative at the beginning of their pregnancy become HIV positive during pregnancy or just afterwards.
Our analysis shows only a part of the global picture. The rates of increase in new infections have been highest in the Eastern European and Central Asia regions (200,000 cases in 2017) [46]. As China did not have available data, it was excluded from our analysis. We are aware that other sources provide different estimates for some countries. For example, much of the WHO country profile data give different figures, although these are from 2016. PEPFAR data also report different figures [24]. It is important to bear in mind that the country reports from 2016 when compared with UNAIDSinfo data from the same year showed variable results [47]. Thus, despite the fact that we have analysed the most up-to-date data on the UNAIDS website, providing a maximally homogenous dataset, estimates must be treated with caution.
In conclusion, higher MTCT rates and numbers of new HIV infections in adults and children, as well as increased HIV/AIDS-related deaths, were found in lower-prevalence rather than higher-prevalence countries, despite lower-prevalence countries accounting for 48% of the global epidemic. These lower-prevalence countries have significantly decreased rates of EID and ART uptake for adults and children. More intensive programmes of diagnosis and treatment are needed in these countries to reduce global new HIV infections to below 500,000 per year by 2020.
References
- 1. UNAIDS 90-90-90: An ambitious treatment target to help end the AIDS epidemic. 2017. Available at: www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf ( accessed March 2019).
- 2. UNAIDS HIV Prevention 2020 Road Map. 2017. Available at: www.unaids.org/sites/default/files/media_asset/hiv-prevention-2020-road-map_en.pdf ( accessed March 2019).
- 3. UNAIDS Progress Report on the Global Plan. 2015. Available at: www.unaids.org/sites/default/files/media_asset/JC2774_2015ProgressReport_GlobalPlan_en.pdf ( accessed March 2019).
- 4. UNAIDS Programme Coordinating Board Background Note: HIV Prevention 2020: a Global Partnership for Delivery. Available at: www.unaids.org/sites/default/files/media_asset/20170613_PCB40_Background-Note_17.14_EN.pdf ( accessed March 2019).
- 5. UNAIDS Get on the fast track: the life-cycle approach to HIV UNAIDS, Geneva, Switzerland, 2016. Available at: www.unaids.org/sites/default/files/media_asset/Get-on-the-Fast-Track_en.pdf ( accessed March 2019).
- 6. UNAIDS Miles to go – closing gaps, breaking barriers, righting injustices. 2018. Available at: https://reliefweb.int/sites/reliefweb.int/files/resources/miles-to-go_en.pdf ( accessed March 2019).
- 7. UNAIDS AIDSinfo. 2018. Available at: aidsinfo.unaids.org/ ( accessed March 2019).
- 8. AVERT Global HIV and AIDS statistics. 2018. Available at: www.avert.org/global-hiv-and-aids-statistics ( accessed March 2019).
- 9. UNAIDS UNAIDS Data 2018. Available at: www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf ( accessed March 2019).
- 10. UNAIDS, The Henry J Kaiser Family Foundation Donor government funding for HIV in low and middle income countries in 2016. 2017. Available at: www.unaids.org/sites/default/files/media_asset/20170721_kaiser_donor_government_funding_hiv.pdf ( accessed March 2019).
- 11. PEPFAR PEPFAR strategy for accelerating HIV/AIDS epidemic control. 2017. Available at: www.pepfar.gov/documents/organization/274400.pdf ( accessed March 2019).
- 12. UNAIDS Ending AIDS. 2017. Available at: www.unaids.org/sites/default/files/media_asset/Global_AIDS_update_2017_en.pdf ( accessed March 2019).
- 13. UNAIDS HIV Prevention among key populations. 2016. Available at: www.unaids.org/en/resources/presscentre/featurestories/2016/november/20161121_keypops ( accessed March 2019).
- 14. Callaghan M, Ford N, Schneider H. A systematic review of task-shifting for HIV treatment and care in Africa. Hum Resour Health 2010; 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Braun R, Catalani C, Wimbush J et al. Community health workers and mobile technology: a systematic review of the literature. PLoS One 2013; 8: e657726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tshuma N, Mosikare O, Yun JA et al. Acceptability of community-based adherence clubs among health facility staff in South Africa: a qualitative study. Patient Prefer Adherence 2017; 11: 1523– 1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilkinson LS. ART adherence clubs: a long-term retention strategy for clinically stable patients receiving antiretroviral therapy. South Afr J HIV Med 2013; 14: 48– 50. [Google Scholar]
- 18. World Health Organization Updated Recommendations on First-Line and Second-Line Antiretroviral Regimens and Post-Exposure Prophylaxis and Recommendations on Early Infant Diagnosis of HIV: Interim Guidance. Geneva: World Health Organization, 2018. [Google Scholar]
- 19. Sabapathy K, Van den Bergh R, Fidler S et al. Uptake of home-based voluntary HIV testing in sub-Saharan Africa: a systematic review and meta-analysis. PLoS Med 2012; 9: e1001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suthar AB, Ford N, Bachanas PJ et al. Towards universal voluntary HIV testing and counselling: a systematic review and meta-analysis of community-based approaches. PLoS Med 2013; 10: e1001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Desai M, Woodhall SC, Nardone A et al. Active recall to increase HIV and STI testing: a systematic review. Sex Transm Dis 2015; 91: 314– 323. [DOI] [PubMed] [Google Scholar]
- 22. Brook G. HIV viral load point-of-care testing: the what, the whys and the wherefores. Sex Transm Infect 2018; 94: 394– 395. [DOI] [PubMed] [Google Scholar]
- 23. Figueroa C, Johnson C, Verster A et al. Attitudes and acceptability on HIV self- testing among key populations: a literature review. AIDS Behav 2015; 19: 1949– 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. UNAIDS Ending AIDS: progress towards the 90–90–90 targets. 2017. Available at: www.unaids.org/sites/default/files/media_asset/Global_AIDS_update_2017_en.pdf ( accessed March 2019).
- 25. Du H, Chi P, Li X. High HIV prevalence predicts less HIV stigma: a cross-national investigation. AIDS Care 2018; 30: 714– 721. [DOI] [PubMed] [Google Scholar]
- 26. Genberg BL, Hlavka Z, Konda KA et al. A comparison of HIV/AIDS-related stigma in four countries: negative attitudes and perceived acts of discrimination towards people living with HIV/AIDS. Soc Sci Med 2009; 68: 2279– 2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shisana O, Rehle T, Simbayi LC et al. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Cape Town: HSRC Press, 2014. [Google Scholar]
- 28. Adams BM. Commercial drone delivery is both exciting and terrifying, but in two East African nations, it's saving lives. 2017. Available at: www.hivplusmag.com/treatment/2017/9/05/watch-fleet-drones-deliver-hiv-drugs-across-tanzania ( accessed March 2019).
- 29. Zukoski AP, Thorburn S. Experiences of stigma and discrimination among adults living with HIV in a low HIV-prevalence context: a qualitative analysis. AIDS Patient Care STDS 2009; 23. [DOI] [PubMed] [Google Scholar]
- 30. Mahajan AP, Sayles JN, Patel VA et al. Stigma in the HIV/AIDS epidemic: a review of the literature and recommendations for the way forward. AIDS 2008; 22 ( suppl 2): S67– S79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brent RJ. The value of reducing HIV stigma. Soc Sci Med 2016; 151: 233– 240. [DOI] [PubMed] [Google Scholar]
- 32. Levi J, Pozniak A, Heath K et al. The impact of HIV prevalence, conflict, corruption, and GDP/capita on treatment cascades: data from 137 countries. J Virus Erad 2018; 4: 80– 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. UNAIDS Location, location: connecting people faster to HIV services. 2013. Available at: www.unaids.org/sites/default/files/media_asset/LocationLocation_en.pdf ( accessed March 2019).
- 34. Datar A. Using PMTCT data to identify HIV epidemic hotspots at subnational levels: lessons learned from the Health Policy Project. [PowerPoint Presentation]. Futures Group [Updated June 2015] Available at: www.slideshare.net/measureevaluation/using-pmtct-data-to-identify-hiv-epidemic-hotspots-at-subnational-levels-lessons-learned-from-the-health-policy-project ( accessed March 2019).
- 35. Wilson D, Taaffe J. Tailoring the local HIV/AIDS response to local HIV/AIDS epidemics In: Disease Control Priorities, 3rd edn ( Holmes KK, Bertozzi S, Bloom BR et al., eds), Washington: World Bank, 2017, pp 157– 178. [Google Scholar]
- 36. Meyer-Rath G, McGillen JB, Cuadros DF et al. Targeting the right interventions to the right people and places: the role of geospatial analysis in HIV program planning. AIDS 2018; 32: 957– 963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wolf RC, Bingham T, Millett G et al. Building the evidence base to optimize the impact of key population programming across the HIV cascade. J Int AIDS Soc 2018; 21 ( suppl 5): e25146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Taylor M, Newman L, Ishikawa N et al. Elimination of mother-to-child transmission of HIV and syphilis (EMTCT): Process, progress, and program integration. PLoS Med 2017; 14: e1002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Unicef Equatorial Guinea: country programme document 2013-2017. Available at: www.unicef.org/about/execboard/files/2012-PL37_Equatorial_Guinea_CPD-final_approved-English.pdf ( accessed March 2019).
- 40. Cook RE, Ciampa PJ, Sidat M et al. Predictors of successful early infant diagnosis of HIV in a rural district hospital in Zambézia, Mozambique. J Acquir Immune Defic Syndr 2011; 56: e104– e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guay L. Early infant diagnosis of HIV: successes, challenges and potential solutions. [PowerPoint Presentation]. Elizabeth Glaser Pediatric AIDS Foundation [Updated July 2010] Available at: www.iasociety.org/HIV-Programmes/Programmes/Industry-Liaison-Forum/Events/Scaling-up-early-infant-diagnosis-of-HIV ( accessed March 2019).
- 42. Finocchario-Kessler S, Gautney BJ, Khamadi S et al. If you text them, they will come: using the HIV infant tracking system to improve early infant diagnosis quality and retention in Kenya. AIDS 2014; 28: S313– S321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cohn J, Whitehouse K, Tuttle J et al. Paediatric HIV testing beyond the context of prevention of mother-to-child transmission: a systematic review and meta-analysis. Lancet HIV 2016; 3: e473– e481. [DOI] [PubMed] [Google Scholar]
- 44. Mahy M, Penazzato M, Ciaranello A et al. Improving estimates of children living with HIV from the Spectrum AIDS Impact Model. AIDS 2017; 31: S13– S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. UNAIDS Reference Group on HIV Estimates Modelling and Projections Report and recommendations from a meeting of the WHO and UNAIDS in collaboration with the UNAIDS Reference Group on Estimates, Modelling and Projections, London, UK, 28-29 October 2015. Available at: www.epidem.org/sites/default/files/reports/Paeds_Report_Oct%202015.pdf ( accessed March 2019).
- 46. GBD 2015 HIV Collaborators Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. Lancet HIV 2016; 3: e361– e387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Levi J, Raymond A, Pozniak A et al. Can the UNAIDS 90-90-90 target be achieved? A systematic analysis of national HIV treatment cascades. BMJ Glob Health 2016; 1: e000010. [DOI] [PMC free article] [PubMed] [Google Scholar]