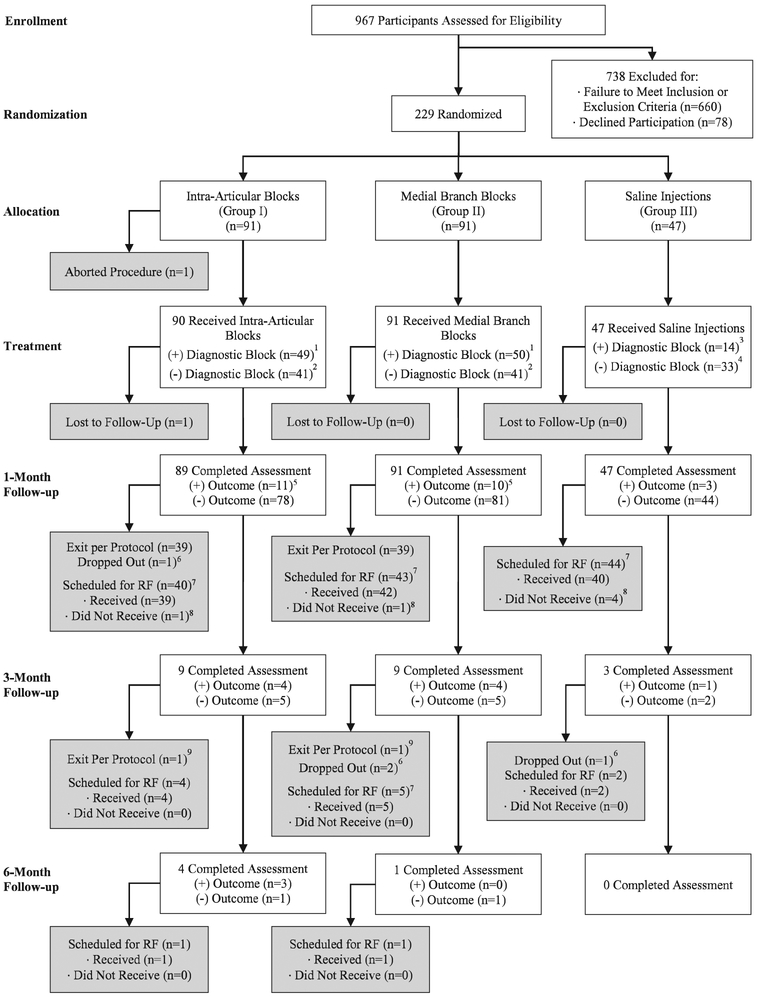
Fig. 2.
Flowchart demonstrating progression through diagnostic and therapeutic facet injection study phase. A positive outcome is defined as 2-point or more decrease in average back pain score coupled with a satisfaction score of 3 out of 5 or higher. (1) Participants with a positive diagnostic block continued the study until a negative follow-up visit, then proceeded with radiofrequency (RF) ablation in an unblinded manner.(2) Participants with a negative diagnostic block continued in the study until a negative follow-up visit, then exited the study. (3) Participants with a positive diagnostic saline injection were treated the same as those in groups I and II (continued in the study until they had a negative follow-up outcome, then proceeded to radiofrequency denervation in an unblinded manner). (4) Participants with a negative diagnostic saline block were unblinded at the point of their negative follow-up visit, then proceeded to radiofrequency denervation. (5) Includes individuals with a negative diagnostic block but positive follow-up outcome that did not exit the study. (6) Includes individuals who were lost to follow-up, chose to exit the study before a negative follow-up visit, or withdrew to receive nonpermitted treatments (e.g., nonradiofrequency denervation treatments or opioid therapy). (7) Scheduled for radiofrequency includes participants who had a negative outcome and a positive block and those who had a positive outcome and a positive block but developed pain before the next follow-up period. (8) Individuals who declined radiofrequency ablation or withdrew from the study before radiofrequency ablation were considered dropouts for the purposes of the study. (9) Includes all those with a negative diagnostic block with initial positive outcome at 1 month, then subsequent negative outcome.