Abstract
Background
Bone morphogenetic protein9 (BMP9) has been reported to have a role in vascular development. However, there is still a lack of information regarding the association between circulating BMP9 levels and cardiovascular disease in humans. The goal of this study is to measure circulating BMP9 concentrations in patients with essential hypertension (HTN), coronary heart disease (CHD) and HTN + CHD, and evaluates the relationship between circulating BMP9 and these cardiovascular diseases.
Methods
A total of 417 individuals were recruited for this cross-sectional study from June 2015 to December 2017. These subjects were screened for HTN and CHD. Circulating BMP9 concentrations were measured by ELISA.
Results
Circulating BMP9 concentrations were significantly low in HTN, CHD and HTN + CHD individuals relative to those of the healthy individuals. Circulating BMP9 correlated negatively with SBP, FIns and HOMA-IR in HTN patients and correlated negatively with FBG and 2 h-BG in CHD patients. In both HTN and CHD patients, circulating BMP9 correlated negatively with BMI, WHR, FAT%, BP and TG. Multivariate logistic regression analysis showed that circulating BMP9 levels were associated with HTN, HTN + CHD and CHD. Individuals with low quartile of circulating BMP9 had a significantly high risk of HTN or/and CHD as compared with those in high quartile.
Conclusions
BMP9 is likely to be a biomarker for cardiovascular disease in humans, and it may play a role in the progression of cardiovascular disease.
Trial registration
Electronic supplementary material
The online version of this article (10.1186/s12872-019-1095-2) contains supplementary material, which is available to authorized users.
Keywords: BMP9, Cytokine, Cardiovascular disease, Hypertension
Background
Essential hypertension (HTN) and coronary heart disease (CHD) are two common diseases in cardiovascular clinics and their major risk factors include genetic, dietary and mental factors [1–4]. Cardiovascular diseases (CVD) cause nearly one third of all deaths worldwide. Coronary heart disease (CHD) accounts for the largest proportion of CVD [5]. Hypertension, dyslipidaemia, obesity and insulin resistance (IR) lead to an increased risk of leaving individuals prone to develop CVD [6]. In those affected by CVD and hypertension are a major contributor to the disease burden [7]. Among Chinese adults aged 35–75 years, nearly half have hypertension, fewer than a third are being treated, and fewer than one in twelve are in control of their blood pressure [8], which lead to increasing risk for CVD [9].
HTN and atherosclerosis are tightly linked metabolic disorders. HTN and CHD are growing global health problems that impact healthcare cost, quality of life and lifespan. Recent years, it has been found that cytokines secreted by hepatocytes, adipocytes and myocytes are signaling protein or extracellular polypeptide. They play an important role in the regulation of inflammation and insulin resistance (IR). Recently, a number of cytokines, such as members of bone morphogenetic proteins (BMPs) superfamily, has been reported to be involved in atherosclerotic lesions and implicated in the pathogenesis of CHD and HTN [10–13].
BMPs are a sub-classification of the transforming growth factor-β (TGF-β) superfamily. BMPs are secreted proteins and are characterized by their ability to induce ectopic bone formation [14, 15]. Recently, BMPs have been shown to be multifunctional cytokines and to have important roles in adipocyte differentiation, energy balance [16, 17] inducing the browning of adipocytes and promoting thermogenesis [18], preventing the formation of lymphatic vessel and human vascular disease [19].
BMP9 and BMP10 have been reported to play an important role in vascular development [20]. In addition, these two members of the BMP family can bind with high affinity to the endothelial-specific receptor activating receptor-like kinase 1 (ALK1) that is involved in vascular diseases [21]. As a secretory protein, BMP9 is also expressed in hepatocytes and secreted into the blood. Circulating BMP9 has also been shown as an important factor to maintain specific endothelial function [22]. In addition, BMP9 and its responsive genes have been reported to regulate vascular endothelial differentiation, promote angiogenesis, inhibit arteriosclerosis, and prevent vascular endothelial cell death [23]. Taken together, all these studies suggest that BMP9 may play an important role in the occurrence and development of vascular diseases, such as CHD and HTN. However, there is still a lack of report regarding the association between serum BMP9 and cardiovascular disease in human.
This study was designed as a cross-sectional cohort study to investigate the changes of circulating BMP9 levels in patients with cardiovascular diseases and its clinical significance. Therefore, we hypothesized that in CHD and HTN patients, circulating BMP9 levels were significantly altered and associated with metabolic disorders and vascular lesions.
Methods
Study design
This is a cross sectional study involving 417 individuals including 78 CHD patients (CHD group), 131 with HTN patients, 87 patients with hypertension complicated with coronary heart disease (CHD) and 121 healthy individuals were recruited in this study from June 2015 to December 2017. Circulating levels of BMP9 were measured in serum samples. The project mainly aimed to measure circulating BMP9 concentrations in patients with essential hypertension (HTN), coronary heart disease (CHD) and HTN + CHD. Further to evaluate the relationship between circulating BMP9 and these cardiovascular diseases. CHD patients were hospitalized with chest discomfort or pain and thus requires investigation with coronary CT angiography CHD was diagnosed by positive coronary angiography or angioplasty (an angiographic evidence of at least one 50% diameter stenosis in one or more coronary arteries) [24–26]. The degree of coronary atherosclerosis depends on the number of vessels in the lesion as a graded variable with significant stenosis. The Gensini score was determined according to the number of segments of stenotic coronary artery [27]. The diagnosis of HTN was based on WHO criteria [systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg], and was confirmed after three visits. Secondary hypertension is determined by clinical, biochemical, hormonal measurements or/and imaging examination. Inclusion criteria for study population included following: 1) age 35–75 years; 2) body mass index (BMI) 17–35 kg/m2. Exclusion criteria included following: 1) acute myocardial infarction; 2) secondary hypertension; 3) type 2 diabetes mellitus (T2DM); 4) lung, liver and kidney disease; 5) cancer; 6) other known major diseases. Individuals with complication were eliminated, such as renal insufficiency, stroke, myocardial infarction and heart failure, etc. 121 age-matched healthy individuals, who had no clinical evidence of any diseases, no taking any medications, and had no family history of T2DM, HTN and CHD, were recruited and were used as the controls. These healthy individuals were recruited from routine medical check-up including coronary CT angiography. CHD in these subjects were excluded by CT coronary angiography (coronary CTA). The study was conducted in accordance with the Declaration of Helsinki, and was approved by the Human Research Ethics Committee of Chongqing Medical University (CHICTR- OCC- 13003185).
Data collection
Anthropometric data were collected by a trained dietician in all participants. Body mass index (BMI) was calculated as weight in kilograms divided by squared height in meters (kg/m2). Waist circumference (WC) was measured midway between the lowest rib and the superior border of iliac crest on midaxillary line. The waist-to-hip ratio (WHR) was calculated by the same researcher. The percentage of body fat (FAT%) was measured by bioelectrical impedance (BIA-101; RJL Systems). Blood pressure (BP) was measured in all participants at least for the rest of 15 min. Blood samples were collected after an 8-10 h fasting and stored at − 80 °C for further measurements. Blood glucose and HbA1c were immediately measured by the glucose oxidase method and anion-exchange HPLC respectively. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated by the following equations: HOMA-IR = fasting insulin (FIns, mU/mL) × fasting blood glucose (FBG, mmol/L) / 22.5 [28]. Insulin was measured by RIA using an ELISA kit. Free fatty acids (FFAs), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) were measured with a commercial kit as previous reported [29].
Circulating levels of BMP9 were measured in serum samples in duplicate with an ELISA kit for human BMP9 according to the manufacturer’s protocol (R&D Systems, Catalogue number #DY3209). The detection line was < 15.60 pg/mL. The intra- and inter-assay coefficients of variation were < 5 and < 10% respectively. The linear range was 15.6–1000 pg/ml. The assay has high sensitivity and excellent specificity for detection of human BMP9 with no cross-reactivity or interference between human BMP9 and other BMPs in circulation.
Statistical analysis
All statistical analyses of this study were performed by A SPSS version 22.0 (SPSS Inc., Chicago, IL). A Kolmogorox-Smirnov test was performed for examining the distribution of data. ANOVA, paired- or unpaired t-test were used for comparison between groups. We used partial correlation coefficients and multivariate regression analyses to examine the association between circulating BMP9 and other variables, respectively. The multivariate logistic regression was used for analyzing the association of BMP9 with HTN or CHD. The Cochran-Armitage trend and row mean scores test were performed to assess the tendency of BMP9 concentration associated with HTN and CHD. The cut-off point of BMP9 level for predicting CHD were given by Receiver operating characteristics (ROC) curves. Sample size was calculated using the following equations: N = [Zα/2 σ/εμ]2 (σ, standard; μ, mean; Zα/2 = 1.96, α = 0.05, ε = 4%). All data were shown as mean ± SD or median (interquartile range). P < 0.05 were considered significant.
Results
Characteristics in study populations
The clinical characteristics of study population were shown in Table 1. The individuals with HTN had higher WHR, BMI, FAT (%), BP, TG, FIns and HOMA-IR than control individuals (P < 0.05 or P < 0.01). In CHD patients, WHR, TG, 2-h blood glucose after glucose overload (2 h-BG), FIns, HbA1c and HOMA-IR were significantly higher, while FFA was lower compared with healthy controls (P < 0.05 or P < 0.01). In addition, in patients with HTN + CHD, WHR, BMI, FAT (%), BP, TG, FIns, 2 h-BG, HbA1c and HOMA-IR were significantly higher, whereas FFA was markedly lower relative to those of controls (P < 0.05 or P < 0.01; Table 1).
Table 1.
Main clinical features and circulating BMP9 levels in the study population
| Variable | Controls (N = 121) | HTN (N = 131) | HTN + CHD (N = 87) | CHD (N = 78) |
|---|---|---|---|---|
| Age (yr) | 50.5 ± 11.7 | 51.5 ± 10.8 | 51.9 ± 10.5 | 52.4 ± 10.8 |
| WHR | 0.88 ± 0.08 | 0.90 ± 0.05* | 0.92 ± 0.06* | 0.93 ± 0.05** |
| BMI (kg/m2) | 23.8 ± 3.4 | 25.1 ± 3.2* | 24.8 ± 3.3* | 23.9 ± 3.1 |
| FAT (%) | 28.7 ± 7.3 | 31.7 ± 7.0** | 31.4 ± 6.9** | 27.1 ± 5.9 |
| SBP (mmHg) | 117.7 ± 13.0 | 147.5 ± 23.1** | 137.8 ± 17.9** | 120.7 ± 10.5 |
| DBP (mmHg) | 74.4 ± 8.9 | 84.4 ± 13.6** | 80.0 ± 10.9** | 73.5 ± 7.3 |
| TC (mmol/L) | 4.52 ± 1.16 | 4.68 ± 0.90 | 4.47 ± 1.21 | 4.43 ± 1.41 |
| TG (mmol/L) | 1.22 (0.83–1.61) | 1.51 (1.07–1.97)** | 1.51 (1.07–2.54)** | 1.28 (0.92–2.19)** |
| LDL-C (mmol/L) | 2.74 ± 0.86 | 2.69 ± 0.76 | 2.96 ± 4.11 | 2.57 ± 1.02 |
| HDL-C (mmol/L) | 1.29 (1.04–1.54) | 1.15 (1.00–1.31) | 1.19 (0.95–1.23) | 1.21 (0.88–1.28) |
| FFA (μmol/L) | 0.53 (0.37–0.64) | 0.56 (0.40–0.76) | 0.41 (0.29–0.58)** | 0.45 (0.33–0.63)** |
| FBG (mmol/L) | 5.28 ± 0.53 | 5.29 ± 0.41 | 5.18 ± 0.45 | 5.18 ± 0.39 |
| FIns (mU/L) | 9.89 (5.38–15.10) | 21.9 (18.4–30.0) ** | 13.7 (7.4–21.5)** | 12.4 (6.5–19.8)** |
| 2 h-BG (mmol/L) | 6.29 ± 1.07 | 6.55 ± 0.84 | 6.62 ± 0.66* | 6.85 ± 1.03* |
| HbA1c (%) | 5.65 ± 0.40 | 5.69 ± 0.39 | 5.73 ± 0.27* | 5.71 ± 0.31* |
| HOMA-IR | 2.45 (1.50–3.60) | 5.04 (4.16–6.83)** | 3.16 (1.61–4.88)** | 2.71 (1.60–4.28)* |
| BMP9 (ng/L) | 123.3 (45.5–178.6) | 53.2 (31.8–62.8) ** | 52.9(27.2–74.7) ** | 55.0 (24.9–71.4)** |
HTN, essential hypertension; CHD, coronary heart disease; WHR, waist hip ratio; BMI, body mass index; FAT%, the percentage of fat in vivo; SBP, systolic blood pressure; DBP, Diastolic blood pressure; TC, total cholesterol; TG, triglyeride; LDL-C, Low-density lipoprotein cholesterol; HDL-C, High-density lipoprotein cholesterol; FFA, free fatty acid; FBG, Fasting blood glucose; FIns, fasting insulin; 2 h-BG, 2-hourblood glucose after glucose overload; HOMA-IR, homeostasis model assessment of insulin resistance. Data are mean ± SD or median (interquartile range). *Data are mean ± SE, Adjustment for age, gender, BMI. *P < 0.05 or **P < 0.01 compared with controls
Circulating BMP9 levels in study population and its correlation with clinical and biochemical parameters
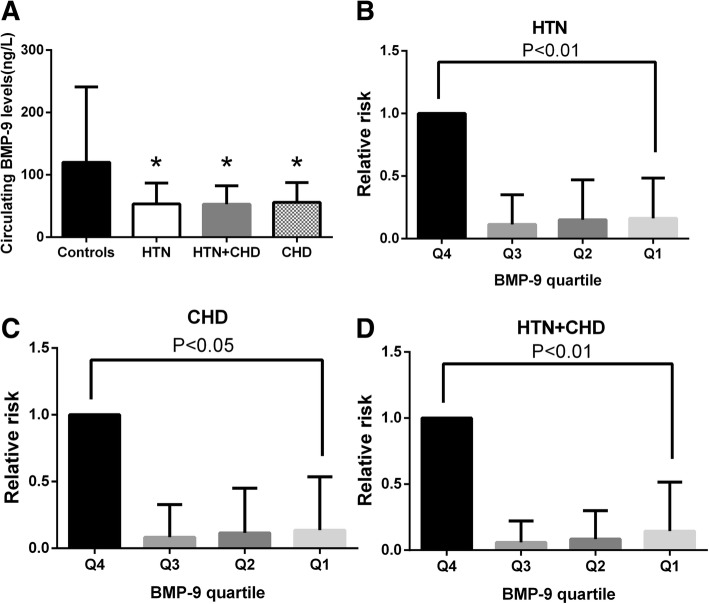
In the current study, we found that circulating BMP9 levels were significantly reduced in HTN, CHD and patients with both HTN and CHD, when compare with healthy controls (all P < 0.01, Fig. 1a and Table 1). After adjusting gender and age, these differences are still significant. Among the three groups of patients, the BMP9 levels in HTN + CHD group were the lowest, but there was no significant difference between the three groups. In partial correlation analysis, we found that the circulating BMP9 correlated negatively with SBP, FIns and HOMA-IR in HTN patients. In both HTN and CHD patients, circulating BMP9 correlated negatively with BMI, WHR, FAT%, BP and TG (P < 0.05 or P < 0.01; Table 2). Finally, in CHD patients, circulating BMP9 correlated negatively with FBG and 2 h-BG (P < 0.05 or P < 0.01; Table 2). These associations remained statistically significant after adjustment for age and sex. In multiple regression analysis of variables, circulating BMP9 levels were independently related to SBP and FFA in HTN patients (both P < 0.01), BMI and FFA in HTN + CHD patients (both P < 0.01) as well as 2 h-BG in CHD patients (P < 0.05, Table 2).
Fig. 1.
Analysis of serum BMP9 by different statistical approaches. a, Circulating BMP9 levels in HTN, CHD and HTN + CHD patients; b, The odds ratio of having HTN in different quartile of BMP9; c, The odds ratio of having CHD in different quartile of BMP9; d, The odds ratio of having HTN + CHD in different quartile of BMP9. *P < 0.05, **P < 0.01 vs. controls or quartile 4
Table 2.
Linear regression analysis of variables associated with circulating BMP9 levels in the study population
| HTN | HTN + CHD | CHD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Simple | Multiple | Simple | Multiple | Simple | Multiple | |||||||
| Variable | R | P | β | P | R | P | β | P | R | P | β | P |
| Age (yr) | −0.086 | 0.201 | 0.289 | <0.01 | −0.021 | 0.856 | ||||||
| BMI (kg/m2) | 0.089 | 0.190 | −0.100 | <0.001 | −9.033 | <0.001 | −0.005 | 0.966 | ||||
| WHR | −0.049 | 0.461 | −0.333 | <0.01 | −0.022 | 0.847 | ||||||
| FAT (%) | −0.085 | 0.205 | −0.515 | <0.001 | 0.158 | 0.166 | ||||||
| SBP (mmHg) | −0.219 | 0.006 | −0.004 | <0.001 | − 0.345 | <0.001 | −0.165 | 0.149 | ||||
| DBP (mmHg) | −0.085 | 0.205 | −0.779 | <0.001 | 0.078 | 0.497 | ||||||
| TG (mmol/L) a | 0.081 | 0.231 | −0.303 | <0.01 | −0.142 | 0.214 | ||||||
| TC (mmol/L) | 0.066 | 0.330 | 0.054 | 0.621 | 0.026 | 0.820 | ||||||
| HDL-C (mmol/L)a | −0.003 | 0.965 | 0.193 | 0.076 | 0.085 | 0.459 | ||||||
| LDL-C (mmol/L) | 0.140 | 0.341 | 0.092 | 0.397 | 0.156 | 0.173 | ||||||
| FFA (mmol/L) a | 0.136 | 0.012 | 0.203 | <0.001 | 0.634 | <0.001 | 0.114 | <0.001 | 0.158 | 0.166 | ||
| FBG (mmol/L) | 0.118 | 0.076 | −0.096 | 0.379 | −0.232 | < 0.05 | ||||||
| 2 h-BG (mmol/L) | −0.134 | 0.051 | 0.009 | 0.938 | −0.10 | < 0.01 | −7.394 | <0.05 | ||||
| FIns (mU/L) a | −0.148 | 0.015 | 0.211 | 0.052 | 0.120 | 0.297 | ||||||
| HbA1c(%) | −0.567 | 0.234 | 0.017 | 0.873 | −0.030 | 0.792 | ||||||
| HOMA-IRa | −0.135 | 0.030 | 0.195 | 0.073 | 0.138 | 0.228 | ||||||
In multiple linear regression analysis, values included for analysis were age, sex, BMI, WHR, FAT, TG, HDL,TC,HOMA-IR,2 h-BG, FFA. aLog transformed before analysis
Association of circulating BMP9 with HTN and CHD
To investigate the relationship between circulating BMP9 and HTN and CHD, we performed multivariate logistic regression analysis. The results showed that circulating BMP9 levels were associated with HTN, HTN + CHD and CHD, even after controlling for anthropometric variables, age, gender, FAT%, blood pressure and lipid profile (Table 3). In addition, to investigate the relationship between BMP9 titer stratification and HTN and CHD, we also performed Row mean scores differ and Cochran-Armitage trend test in all study population. The results revealed that the decreasing levels of BMP9 showed a significant linear trend and were independently associated with HTN and CHD, when the concentration was analyzed (Table 4).
Table 3.
Association of circulating BMP9 with HTN and CHD in fully adjusted models
| HTN | HTN + CHD | CHD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P-value | OR | 95%CI | P-value | OR | 95%CI | P-value | |
| Model 1 | 0.983 | 0.978–0.989 | <0.001 | 0.984 | 0.976–0.992 | <0.001 | 0.988 | 0.981–0.996 | <0.01 |
| Model 2 | 0.983 | 0.978–0.989 | <0.001 | 0.987 | 0.979–0.995 | <0.001 | 0.988 | 0.980–0.996 | <0.01 |
| Model 3 | 0.983 | 0.978–0.989 | <0.001 | 0.990 | 0.982–0.998 | <0.05 | 0.988 | 0.980–0.996 | <0.01 |
| Model 4 | 0.985 | 0.978–0.991 | <0.001 | 0.984 | 0.974–0.994 | <0.001 | 0.987 | 0.979–0.995 | <0.01 |
Model1, adjusted age, gender; Model2, adjusted age, gender, WHR; Model3, adjusted age, gender, WHR BMI, FAT, FBG; Model 4, adjusted age, gender, WHR BMI, FAT, FBG, lipid profile. Results of multivariate logistic regression analysis are presented as the odds ratio (OR) of being in HTN, HTN + CHD and CHD status increase in circulating BMP9
Table 4.
Row mean scores differ and Cochran-Armitage trend analysis of the impact of circulating BMP9 levels on HTN and CHD
| HTN | HTN + CHD | CHD | ||||
|---|---|---|---|---|---|---|
| Model adjusted | X 2 | P-value | X 2 | P-value | X 2 | P-value |
| Row Mean Scores Test | 55.260 | <0.001 | 7.451 | <0.01 | 5.275 | <0.05 |
| Cochran-Armitage Trend Test | 3.6421 | <0.001 | 2.736 | <0.01 | 2.303 | <0.05 |
Values shown are cut-offs of circulating BMP9 levels of all subjects. Adjusted for age, sex, BMI, WHR, blood pressure, TG, TC, LDL-C and HDL-C
The relative risk of prevalent of cardiovascular diseases for circulating BMP9 levels
According to the concentration of BMP9 in HTN, CHD and HTN + CHD patients, it is divided into quartile (quartile 1, < 31.80 ng/L, quartile 2, 31.81–46.44 ng/L, quartile 3, 46.45–62.80 ng/L, quartile 4, > 62.80 ng/L for HTN; quartile 1, < 27.24 ng/L, quartile 2, 27.25–42.25 ng/L, quartile 3, 42.26–74.71 ng/L, quartile 4, > 74.71 ng/L for HTN + CHD and quartile 1, < 24.98 ng/L, quartile 2, 24.99–54.95 ng/L, quartile 3, 54.96–70.86 ng/L, quartile 4, > 70.86 ng/L, respectively). As shown in Fig. 1b-d, individuals in low quartile of circulating BMP9 had a significantly high risk of HTN or CHD or both HTN and CHD compared with those of high quartile. These changes still exist, even after the adjustment of age, sex, BMI and WC.
To evaluate the relationship between BMP9 and CHD or HNT, respectively, circulating BMP9 concentrations were further stratified. We found that each stratified concentration was correlated with the risk of CHD or HNT, respectively. With per standard deviation equivalent (1-SD) decrease of BMP9 concentrations, the risk of CHD or HTN increased significantly (Additional file 1: Table S1).
To explore the predictive value of circulating BMP9 for CHD, we performed the ROC curves analysis. This analysis showed that the area under the ROC curves (AUC) was 0.686 (P < 0.001) with a sensitivity of 62.8% and specificity of 44.9% for CHD (Fig. 2), and the best cutoff values for circulating BMP9 to predict CHD was 57.3 ng/L.
Fig. 2.
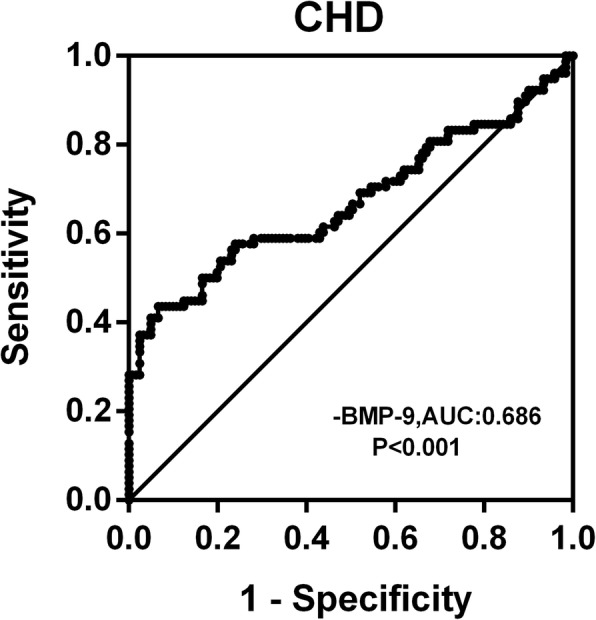
ROC curve analysis was performed for the prediction of CHD
Discussion
HTN is a common cardiovascular disease, and the causal factors include genetic, dietary and mental factors [30–32]. The main risk of HTN is a continuous increase in blood pressure, thereby increasing the burden of the left ventricle and eventually leading to CHD [33]. Therefore, it is important to explore the common biomarkers between HTN and CHD.
This cross-sectional study was performed in a community-based middle to older aged Chinese population. Up to now, few studies have reported circulating BMP9 levels in both HTN and CHD patients and the association of circulating BMP9 with HTN and CHD risk in humans. In the current study, we found that fasting BMP9 levels were lower in HTN, CHD or HTN + CHD patients than those of healthy controls. These results are similar to our previous report in T2DM patients [34]. Therefore, current and previous results indicated that BMP9 could have a role in linking metabolic disorder and arterial stiffness, and have an impact on the pathophysiology of IR and arteriosclerosis-related diseases. Although the nature of the current study does not permit us to determine the cause of decreased circulating BMP9 in CHD and HTN patients, we speculate that the decrease of BMP9 in patients with cardiovascular disease might be a defensive response to metabolic stress and arteriosclerosis. Therefore, whether increasing circulating BMP9 level can improve lipid metabolism, stabilize arterial plaque and improve vascular endothelial function should be further studied.
In the current study, circulating BMP9 correlated negatively with FBG and 2 h-BG in CHD patients, while 2 h-BG was an independently related factor influencing circulating BMP9 levels. Therefore, in CHD patients, circulating BMP9 levels were mainly affected by blood glucose levels, suggesting an association between BMP9 and glucose metabolism. In patients with both HTN and CHD, circulating BMP9 correlated negatively with BP, parameters of adiposity (BMI, WHR and FAT %) and parameters of fat metabolism (TG and FFA). BMI and FFA were independently related factors for circulating BMP9 levels. Therefore, circulating BMP9 was mainly impacted by obesity and the disorder of lipid metabolism in these patients.
The analyses employing 3 quartiles of circulating BMP9 demonstrated that patients with lower quartiles of BMP9 were more likely to develop HTN, CHD and HTN + CHD when compared to those in the highest quartile. To assess the diagnostic capacity of circulating BMP9 in CHD and the coexistence of CHD, ROC curve analysis was performed. The ROC analysis further showed that BMP9 was associated with CHD. BMP9 could be a discriminative performance measure as an indicator of cardiovascular disease. The cut-off point of ROC curve indicated sensitivity and specificity values between 40 and 60%, which minimizes false-positive and false-negative cases.
Therefore, circulating BMP9 has certain clinical value in the diagnosis of cardiovascular diseases. Based on these results, we postulate that BMP9 may serve as a biomarker for the progress of cardiovascular diseases.
Limitations
1). This was a cross-sectional study with a single center on Chinese patients, it can’t determine a cause-effect relationship between circulating BMP9 and HTN as well as CHD; 2) Our study did not examine the sources of BMP9; 3) The size of the sample is relatively small considering that it is divided into four study groups, one independent from the other, and each with a different number of patients. Therefore, clinical application may be limited; 4) Our study was based on single measurements of serum BMP9, which may not reflect the changes of circulating BMP9 over time. Therefore, it would be interesting to take a new measurement of BMP9 after a few months; 5) As the population studied is exclusively Chinese, it cannot be applied to the general population. Therefore, serial alternation of serum BMP9 should be measured at different stages of these patients to clarity the role of BMP9 at the onset of HTN and CHD. Finally, the study individuals were from Chinese populations which have similar lifestyles. Thus, these data may not be directly applicable to other races.
Conclusions
Our study demonstrated that circulating BMP9 levels are decreased in HTN, CHD and HTN + CHD individuals, and circulating BMP9 correlated with the prevalence rate of cardiovascular diseases. We postulate that the risks of HTN, CHD and HTN + CHD increase as circulating BMP9 concentration increase. Therefore, our data suggest that BMP9 plays a role in the pathophysiology of HTN and CHD, and circulating BMP9 is likely to be a biomarker for the progress of HTN, CHD and HTN + CHD.
Additional file
Table S1. The risk of prevalent of CHD or HNT according to quartiles for serum BMP9 concentrations. (DOCX 16 kb)
Acknowledgements
Not applicable.
Funding
This work was supported by research grants from the National Natural Science Foundation of China (81873658 and 81300670) for data collection, collection and transportation of the blood samples and the processing of samples in the laboratory.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- 2 h-BG
2-h blood glucose after glucose overload
- BMI
Body mass index
- BP
Blood pressure
- FAT %
The percentage of body fat
- FBG
Fasting blood glucose
- FFA
Free fatty acid
- Fins
Fasting insulin
- HOMA-IR
Homeostasis model assessment of insulin resistance
- SBP
Systolic blood pressure
- TG
Triglyceride
- WHR
Waist hip ratio
Authors’ contributions
RL, XL, WH and DP contributed to data collection and analysis. GY and MT designed the analytic strategy and drafted the manuscript. HL revised and edited the manuscript. MT and DZ were the guarantors of this work and, as such, had full access to all the data in this study and take responsibility for the integrity of the data and accuracy of data analysis. All authors edited and approved the final manuscript.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki, and was approved by the Human Research Ethics Committee of Chongqing Medical University (CHICTR-OCC-13003185). All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors of the manuscript do not have any closely related papers or manuscripts that have been submitted or published elsewhere and declare that they do not have any competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rui Liu, Email: 317085605@qq.com.
Wenjing Hu, Email: 273297582@qq.com.
Xiaoqiang Li, Email: 404440648@qq.com.
Danlan Pu, Email: 22269167@qq.com.
Gangyi Yang, Email: gangyiyang@163.com.
Hua Liu, Email: hliu@umc.edu.
Minghong Tan, Email: 13983606910@139.com.
Danping Zhu, Email: zdp790203@163.com.
References
- 1.De Hert M, Detraux J, Vancampfort D. The intriguing relationship between coronary heart disease and mental disorders. Dialogues Clin Neurosci. 2018;20(1):31–40. doi: 10.31887/DCNS.2018.20.1/mdehert. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiNicolantonio JJ, Lucan SC, O'Keefe JH. The Evidence for Saturated Fat and for Sugar Related to Coronary Heart Disease. Prog Cardiovasc Dis. 2016;58(5):464–472. doi: 10.1016/j.pcad.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garfinkle MA. Salt and essential hypertension: pathophysiology and implications for treatment. J Am Soc Hypertens. 2017;11(6):385–391. doi: 10.1016/j.jash.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Wise IA, Charchar FJ. Epigenetic Modifications in Essential Hypertension. Int J Mol Sci. 2016;17(4):451. doi: 10.3390/ijms17040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong ND. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat Rev Cardiol. 2014;11(5):276–289. doi: 10.1038/nrcardio.2014.26. [DOI] [PubMed] [Google Scholar]
- 6.Yatsuya H, Li Y, Hilawe EH, Ota A, Wang C, Chiang C, et al. Global trend in overweight and obesity and its association with cardiovascular disease incidence. Circ J. 2014;78(12):2807–2818. doi: 10.1253/circj.CJ-14-0850. [DOI] [PubMed] [Google Scholar]
- 7.GBD 2015 Risk Factors Collaborators Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu J, Lu Y, Wang X, Li X, Linderman GC, Wu C, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 1.7 million adults in a population-based screening study (China PEACE Million Persons Project) Lancet. 2017;390(10112):2549–2558. doi: 10.1016/S0140-6736(17)32478-9. [DOI] [PubMed] [Google Scholar]
- 9.Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, Horwich T, et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the american heart association. Circulation. 2016;134(23):e535–ee78. doi: 10.1161/CIR.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 10.Lau WB, Ohashi K, Wang Y, Ogawa H, Murohara T, Ma XL, Ouchi N. Role of Adipokines in Cardiovascular Disease. Circ J. 2017;81(7):920–928. doi: 10.1253/circj.CJ-17-0458. [DOI] [PubMed] [Google Scholar]
- 11.Tsai JP, Wang JH, Chen ML, Yang CF, Chen YC, Hsu BG. Association of serum leptin levels with central arterial stiffness in coronary artery disease patients. BMC Cardiovasc Disord. 2016;16:80. doi: 10.1186/s12872-016-0268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long L, Ormiston ML, Yang X, Southwood M, Gräf S, Machado RD, et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med. 2015;21(7):777–785. doi: 10.1038/nm.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derwall M, Malhotra R, Lai CS, Beppu Y, Aikawa E, Seehra JS, et al. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. 2012;32(3):613–22. [DOI] [PMC free article] [PubMed]
- 14.Böttcher Y, Unbehauen H, Klöting N, Ruschke K, Körner A, Schleinitz D, et al. Adipose tissue expression and genetic variants of the bone morphogenetic protein receptor 1Agene (BMPR1A) are associated with human obesity. Diabetes. 2009;58(9):2119–2128. doi: 10.2337/db08-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller AF, Harvey SA, Thies RS, Olson MS. Bone morphogenetic protein-9. An autocrine/paracrine cytokine in the liver. J Biol Chem. 2000;275(24):17937–17945. doi: 10.1074/jbc.275.24.17937. [DOI] [PubMed] [Google Scholar]
- 16.Tobin JF, Celeste AJ. Bone morphogenetic proteins and growth differentiation factors as drug targets in cardiovascular and metabolic disease. Drug Discov Today. 2006;11(9–10):405–411. doi: 10.1016/j.drudis.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Schulz TJ, Tseng YH. Emerging role of bone morphogenetic proteins in adipogenesis and energy metabolism. Cytokine Growth Factor Rev. 2009;20(5–6):523–531. doi: 10.1016/j.cytogfr.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454(7207):1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshimatsu Y, Lee YG, Akatsu Y, Taguchi L, Suzuki HI, Cunha SI, et al. Bone morphogenetic protein-9 inhibits lymphatic vessel formation via activin receptor-like kinase 1 during development and cancer progression. Proc Natl Acad Sci U S A. 2013;110(47):18940–18945. doi: 10.1073/pnas.1310479110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Salmon RM, Jiang H, Morrell NW. Regulation of the ALK1 ligands, BMP9 and BMP10. Biochem Soc Trans. 2016;44(4):1135–1141. doi: 10.1042/BST20160083. [DOI] [PubMed] [Google Scholar]
- 21.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109(5):1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- 22.Levet S, Ouarné M, Ciais D, Coutton C, Subileau M, Mallet C, et al. BMP9 and BMP10 are necessary for proper closure of the ductus arteriosus. Proc Natl Acad Sci U S A. 2015;112(25):E3207–E3215. doi: 10.1073/pnas.1508386112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tachida Y, Izumi N, Sakurai T, Kobayashi H. Mutual interaction between endothelial cells and mural cells enhances BMP9 signaling in endothelial cells. Biol Open. 2017;6(3):370–380. doi: 10.1242/bio.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cury RC, Abbara S, Achenbach S, Agatston A, Berman DS, Budoff MJ, et al. Coronary artery disease – reporting and data system (CAD-RADS):an expert consensus document of SCCT, ACR and NASCI: endorsed by the ACC. J Am Coll Cardiol Img. 2016;9(9):1099–1113. doi: 10.1016/j.jcmg.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Sorbets E, Steg PG, Young R, Danchin N, Greenlaw N, Ford I, et al. β-Blockers, calcium antagonists, and mortality in stable coronary artery disease: an international cohort study. Eur Heart J. 2018; Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 26.Ibe S, Kishimoto Y, Niki H, Saita E, Umei T, Miura K, et al. Associations between plasma nesfatin-1 levels and the presence and severity of coronary artery disease. Heart Vessels. 2019 [Epub ahead of print]. [DOI] [PubMed]
- 27.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51(3):606. doi: 10.1016/S0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 28.AlbaredaM R-EJ, Murugo M, de Leiva A, Corcoy R. Assessment of insulin sensitivity and beta-cell function from measurements in the fasting state and during an oral glucose tolerance test. Diabetologia. 2000;43(12):1507–1511. doi: 10.1007/s001250051561. [DOI] [PubMed] [Google Scholar]
- 29.Li K, Liao X, Wang K, Mi Q, Zhang T, Jia Y, et al. Myonectin predicts the development of type 2 diabetes. J Clin Endocrinol Metab. 2018;103(1):139–147. doi: 10.1210/jc.2017-01604. [DOI] [PubMed] [Google Scholar]
- 30.Brott BC. Prevention of myocardial stunning during percutaneous coronary interventions: novel insights from pre-treatment with glucagon-like peptide-1. JACC Cardiovasc Interv. 2015;8(2):302–304. doi: 10.1016/j.jcin.2014.12.218. [DOI] [PubMed] [Google Scholar]
- 31.Fiechter M, Fuchs TA, Stehli J, Jacobs S, Falk V, Kaufmann PA. Reversible true myocardial hibernation. Eur Heart J. 2013;34(9):648. doi: 10.1093/eurheartj/ehs414. [DOI] [PubMed] [Google Scholar]
- 32.Page BJ, Banas MD, Suzuki G, Weil BR, Young RF, Fallavollita JA, et al. Revascularization of chronic hibernating myocardium stimulates myocyte proliferation and partially reverses chronic adaptations to ischemia. J Am Coll Cardiol. 2015;65(7):684–697. doi: 10.1016/j.jacc.2014.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng Y, Zong L, Zhang Z, Han Y, Wang Y. Evaluation of changes in left ventricular structure and function in hypertensive patients with coronary artery disease after PCI using real-time three-dimensional echocardiography. Exp Ther Med. 2018;15(2):1493–1499. doi: 10.3892/etm.2017.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo Y, Li L, Xu X, Wu T, Yang M, Zhang C, et al. Decreased circulating BMP-9 levels in patients with type 2 diabetes is a signature of insulin resistance. Clin Sci (Lond) 2017;131(3):239–246. doi: 10.1042/CS20160543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The risk of prevalent of CHD or HNT according to quartiles for serum BMP9 concentrations. (DOCX 16 kb)
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.