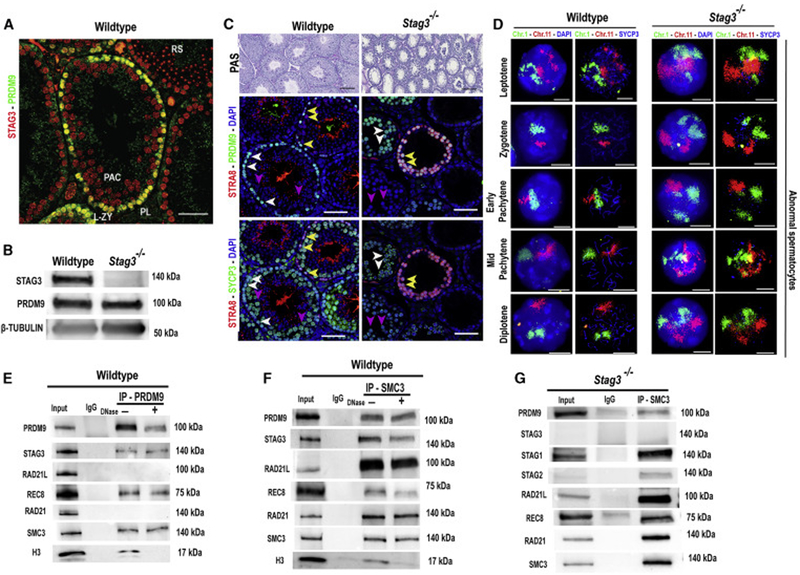
Figure 1. STAG3-associated cohesin complexes co-express and interact with PRDM9 in mouse spermatocytes.
(A) Immunofluorescence staining of a wildtype testis cross-section probed with PRDM9 (green) and STAG3 (red) antibodies. PRDM9 and STAG3 co-express in pre-leptotene (PL, white arrow heads), leptotene and zygotene spermatocytes (L-ZY, magenta arrow heads). No PRDM9 is detected in pachytene spermatocytes (PAC, turquoise arrow heads) or round spermatids (RS). Scale bar=100μm.
(B) Immunoblot showing expression of STAG3, PRDM9 and β-TUBULIN (loading control) in juvenile (12 d postpartum) testes from wildtype and Stags−/− males. 50 μg of protein extract was loaded per lane.
(C) Top panel: PAS-stained histological sections of wildtype (left) and Stag3−/− (right) testis at 8 weeks of age. The middle and the bottom panels are testis sections stained with different antibody combinations. Middle panel: Immunofluorescence staining of histological sections of wildtype and Stag3−/− testis stained with anti-PRDM9 (green), and anti-STRA8 (red); DNA is stained with DAPI (blue). Bottom panel: The same wildtype and Stag3−/− testis histological sections immunolabeled using anti-SYCP3 (green) and anti-STRA8 (red); DNA is stained with DAPI (blue). STRA8, SYCP3 and PRDM9 co-express in pre-leptotene and leptotene cells (yellow arrow heads), and in zygotene (-like) spermatocytes (white arrow heads) SYCP3 and PRDM9 co-express. In pachytene (-like) spermatocytes (blue arrow heads), SYCP3 is detected, but no PRDM9 is seen. Scale bars=100μm.
(D) Wildtype (two left columns) and Stag3−/− (two right columns) spermatocyte chromatin spreads immunolabeled with antibodies against the SC lateral element protein SYCP3 (blue) and hybridized with whole-chromosome FISH paint probes for Chr 1 (green) and Chr 11(red); DNA is stained with DAPI (blue). Note larger and less distinct chromosome territories, as well as separated homologs, in mutant panels (right). Scale bars=10μm.
(E&F) Co-immunoprecipitation of PRDM9 with chromosomal axis proteins STAG3, REC8, and SMC3 from wildtype 14-dpp spermatocytes (E), confirmed by reverse co-immunoprecipitation with anti-SMC3 antibody (F). Input extract, 20 μg (2.5 μg in the RAD21,SMC3 and H3 blots); lane 2, 20 μg extract coIP with nonimmune IgG; αPRDM9 and αSMC3 (F), 20 μg extract coIP with the respective antibody, either not-treated ("−") or treated ("+") with DNase. Histone H3 IP was used to detect efficiency of DNase treatment. The antibodies used to detect specific proteins are shown on the left and the relevant molecular weights are shown on right of each blot. For quantified values of protein bands please refer to Table S1 and S2.
(G) Co-immunoprecipitation of PRDM9 and cohesin proteins from Stag3−/− spermatocytes. This analysis confirmed that PRDM9 can be associated with other cohesin subunits in absence of STAG3, and demonstrates the protein-protein interaction of REC8 and RAD21L with other cohesin subunits in absence of STAG3 previously reported [7]. Input extract, 20 μg (2.5 μg in the STAG1, STAG2, RAD21 and SMC3 blots); lane 2, 20 μg extract coIP with nonimmune IgG and lane 3, 20 μg extract coIP with SMC3 antibody. The relevant molecular weights are shown on right of each blot.