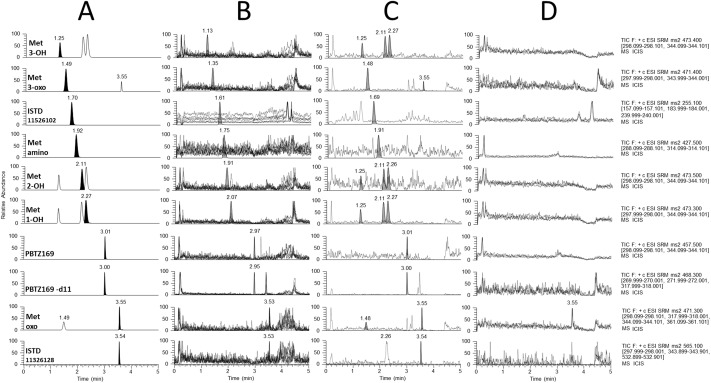
Fig 2.
(A) UHPLC-MS/MS separation of PBTZ169, six in vitro known metabolites and internal standards in spiked human plasma (calibration sample at 500 ng/mL). Each targeted compound is reported as a black chromatographic peak at the selected m/z transition (i.e. to distinguish them from position isomers signals). (B) Selectivity for matrix interferences: overlaid UHPLC-MS/MS profiles (dotted lines) of methanolic extracts from ten blank human plasma. (C) Selectivity for analytical interferences: UHPLC-MS/MS profiles for checking mutual interferences between analytes and ISTD. The background profiles (dotted lines) reported in the analytes LC-MS/MS traces are obtained after precipitation of a human blank plasma with processed with only ISTD solution at 40 ng/mL, whereas background profiles depicted in the ISTD LC-MS/MS traces are obtained after precipitation of the highest calibrator level (2000 ng/mL) with pure MeOH. For (B) and (C), retention times and chromatographic LC-MS/MS profiles of PBTZ169, active metabolites, Met-amino and ISTD obtained during experiments were superimposed for interpretation. (D) Carryover: overlaid UHPLC-MS/MS profiles (n = 3) obtained for the first injection of blank solvent (MeOH) after the highest calibration sample (2000 ng/mL).