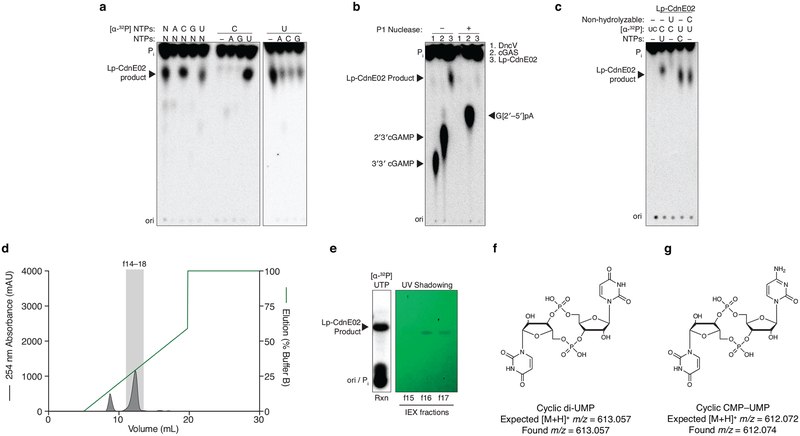
Extended Data Figure 7 |. Detailed biochemical analysis of Lp-CdnE02.
a-, Biochemical deconvolution of Lp-CdnE02 (CD-NTase057) as in Fig. 1c, demonstrates specific synthesis of cyclic dipyrimidine products. Data are representative of 3 independent experiments.
b, Nuclease sensitivity of the Lp-CdnE02 product, as described in Extended Data Fig. 1d. Data are representative of 3 independent experiments.
c, Incubation of Lp-CdnE02 with nonhydrolyzable nucleotides, as described in Extended Data Fig. 2. Nonhydrolyzable UTP completely blocks the reaction, indicating the first step requires attack of the α-P from UTP. However, the product formed when nonhydrolyzable CTP is present cannot be distinguished from c-di-UMP in this assay, and it is unclear if the reaction proceeds through a pppCpU reaction intermediate. Data are representative of 3 independent experiments.
d, Anion exchange chromatography of a Lp-CdnE02 reaction with UTP and CTP, eluted with a gradient of Buffer B (2 M ammonium acetate) by FPLC. Individual fractions were concentrated prior to pooling for further analysis.
e, Anion exchange chromatography (IEX) fractions from d were separated by silica TLC, visualized by UV shadowing, and compared to a radiolabeled reaction to confirm the appropriate peak. Fractions were pooled and concentrated prior to MS analysis.
f, MS confirmed synthesis of c-di-UMP as the major product of Lp-CdnE02.
g, MS confirmed synthesis of cCMP–UMP as a minor product of Lp-CdnE02.