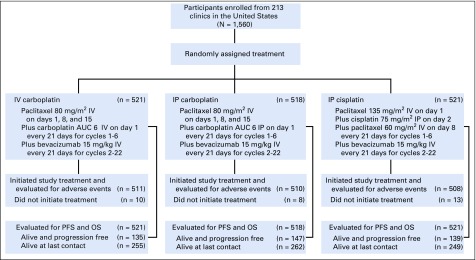
FIG 1.
CONSORT diagram that accounts for all participants and the chemotherapy regimen arms to which they were randomly assigned. Crossover during treatment to the IV arm occurred in 16% of those randomly assigned to the IP carboplatin arm and 28% of those randomly assigned to the IP cisplatin arm. Taxotere was substituted for paclitaxel in 7% of those in the IV and IP carboplatin arms and 5% of those in the IP cisplatin arm. There was discontinuation of bevacizumab during the concurrent chemotherapy administration of the first six cycles in 15% of participants in the IV and IP carboplatin arms and 30% of participants in the IP cisplatin arm. The median number of cycles of bevacizumab was 20 for those in the IV carboplatin arm, 19 in the IP carboplatin arm, and 17 in the IP cisplatin arm. Reason for discontinuation of study regimen was completion of therapy in 51% of participants in the IV carboplatin arm, 49% in the IP carboplatin arm, and 45% in the IP cisplatin arm. Toxicity caused discontinuation in 24%, 28%, and 29%, respectively, and refusal was 6%, 6%, and 9%, respectively. Disease progression or death was cause for discontinuation in 16%, 13%, and 13%, respectively. The delivery of six cycles of any platinum agent was 90% in the IV and IP carboplatin arms and 84% in the IP cisplatin arm. Day 8 paclitaxel was delivered to 66% of participants in the IV or IP carboplatin arms for all six cycles and only 59% in the IP cisplatin arm. Four cycles of day 8 paclitaxel was received by 85% of participants in the IV and IP carboplatin arms and 80% of those in the IP cisplatin arm. In the IV and IP carboplatin arms, only 40% of participants received day 15 paclitaxel through six cycles, and 60% received it through four cycles, whereas 80% received two cycles. AUC, area under the curve; OS, overall survival; PFS, progression-free survival.