Abstract
Background and Objective
Chronic low back pain (CLBP) is highly prevalent, with a substantial psychosocial burden. Pain has both sensory and affective components. The latter component is a significant driver of disability and psychiatric comorbidity but is often inadequately treated. Previously we reported that noninvasive transcranial direct current stimulation (tDCS) may modulate pain-associated affective distress. Here we tested whether 10 daily tDCS sessions aimed to inhibit the left dorsal anterior cingulate cortex (dACC), a region strongly implicated in the affective component of pain, would produce selective reduction in pain-related symptoms.
Methods
In this multisite, double-blinded, randomized placebo-controlled trial (RCT), 21 CLBP patients received 10 weekday sessions of 2-mA active tDCS or sham (20 minutes/session). A cathodal electrode was placed over FC1 (10–20 electroencephalography coordinates), and an identical anodal return electrode was placed over the contralateral mastoid. Participants rated pain intensity, acceptance, interference, disability, and anxiety, plus general anxiety and depression.
Results
Regression analysis noted significantly less pain interference (P =0.002), pain disability (P =0.001), and depression symptoms (P =0.003) at six-week follow-up for active tDCS vs sham. Omnibus tests suggested that these improvements were not merely due to baseline (day 1) group differences.
Conclusions
To our knowledge, this is the first double-blinded RCT of multiple tDCS sessions targeting the left dACC to modulate CLBP’s affective symptoms. Results are encouraging, including several possible tDCS-associated improvements. Better-powered RCTs are needed to confirm these effects. Future studies should also consider different stimulation schedules, additional cortical targets, high-density multi-electrode tDCS arrays, and multimodal approaches.
Keywords: Transcranial Direct Current Stimulation, tDCS, Chronic Low Back Pain, CLBP, Dorsal Anterior Cingulate Cortex, dACC
Introduction
Chronic low back pain (CLBP) is a massive burden on patients, families, and the health care system, having an annual prevalence of 15%–45% [1]. Defined as lasting three months or more [2], it includes both sensory and emotional (affective) components [3]. In CLBP, this emotional component drives much of the morbidity and disability, including maladaptive avoidance behaviors [4] and serious psychiatric sequelae [5]. Although the process of pain “chronification” remains poorly understood, it appears to involve altered brain structure and function [6–8]. The current standard of clinical care for CLBP primarily consists of prescription analgesic medications such as opioids and nonsteroidal antiinflammatory drugs (NSAIDs) or nonmedication modalities such as physical therapy. In addition to the obvious pitfalls of chronic opioid use [9–12], such drugs do not treat CLBP’s affective symptoms. Cognitive behavioral therapy (CBT) can effectively treat these symtpoms [13–18], but many patients encounter access barriers. Easily deployed, low-cost, nonopioid treatments targeting CLBP’s affective symptoms are needed.
The anterior cingulate cortex (ACC) participates in human pain processing, comprising the medial pain pathway with the medial thalamus, anterior insula, and posterior parietal cortex [19]. Along with primary and secondary somatosensory cortices, the ACC is associated with “second pain sensation” [20] and with chronic pain [21]. The region has one of the highest densities of opioid receptors in the human brain and is a primary site of action for opioidergic agents [22]. For over half a century, cingulotomy, the neurosurgical destruction of ACC, has been used to relieve intractable pain, with patients reporting reduced pain-related distress even though the reported intensity did not necessarily decrease [23–27]. Analogous results have been found with deep brain stimulation (DBS) of the dorsal ACC (dACC), demonstrating improvement in the affective component of pain [28] that is durable [29].
Noninvasive brain stimulation (NIBS) is a burgeoning research area. Transcranial direct current stimulation (tDCS) involves applying a fixed low-intensity electric current (e.g., 1–2 mA) to the brain via electrodes placed on the scalp, between which an electric field is established [30]. In contrast to other NIBS modalities such as electroconvulsive therapy (ECT) or transcranial magnetic stimulation (TMS), tDCS alone does not fully depolarize neurons, but rather increases or decreases the likelihood for neurons to “fire” action potentials depending on the direction of current flow [31,32]. Thus, neurons of a brain region beneath a cathodal electrode would be relatively hyperpolarized and less likely to produce action potentials, whereas neurons of a brain region beneath an anodal electrode would be relatively depolarized and more likely to produce action potentials [32]. An advantage of tDCS is that current can flow through deeper brain structures. Although tDCS has been shown to modulate the intensity (sensory component) of chronic forms of pain [33–37], there has been little work testing if tDCS can modulate the affective component of pain. The authors’ prior work suggests that tDCS with a cathodal electrode targeting the left dACC (with an identically sized anodal return electrode placed on the contralateral mastoid) may improve pain distress tolerance in healthy volunteers [38], as compared with tDCS with anodal electrode targeting the left dACC and cathodal electrode on the contralateral mastoid. However, there is a lack of randomized and double-blinded clinical studies that investigate tDCS effects in chronic pain populations.
We therefore conducted a placebo-controlled, double-blinded, randomized controlled trial (RCT) in CLBP patients. We wished to study the effects of multiple sessions of tDCS—using an electrode montage comprised of a cathodal electrode intended to target the left dACC (and thus decrease the region’s activity) and an identically sized anodal return electrode over the contralateral mastoid—on measures of the sensory and emotional components of CLBP. We anticipated that 10 daily sessions of tDCS would be well tolerated in a clinical CLBP population, would reduce pain-related interference and distress, and would increase pain acceptance—but not necessarily change rated pain intensity.
Methods
Participants
We recruited and consented 30 participants (seven female) with CLBP via direct clinician referrals and posted advertisements at the Providence Veterans Affairs Medical Center (PVAMC) and Butler Hospital, both located in Providence, Rhode Island. Study protocols were approved by the institutional review boards of both hospitals, using identical procedures at both sites. The study was registered with ClinicalTrials.gov (NCT02771990, NCT02768129). Potential participants received a brief telephone prescreen to verify basic eligibility, then were scheduled for a full in-person screening, at which time we also obtained written informed consent and prescription opioid status. Eligible participants were financially compensated for their participation. We did not use our earlier results [38] to perform a sample size calculation for the present study because our prior work was performed in healthy volunteers, was not placebo-controlled, and compared only one session each of two active stimulation conditions.
We accepted participants older than 18 years of age who were on stable medications for at least one month. Participants were required to have clinician-diagnosed CLBP of at least six months’ duration in the lumbar region that was present more than half the days of the month and on average at a moderate level of severity in the last month by scoring at least 4 on an 11-point visual analog scale, the Defense and Veterans Pain Rating Scale (DVPRS) [39]. Participants must have had at least one trial of physician-recommended medication (e.g., acetaminophen, NSAIDS, skeletal muscle relaxants). Doses of preexisting opioid or nonopioid pain medication must have been stable for at least one month, where present.
Potential participants were excluded if they had a lifetime diagnosis of bipolar disorder or chronic psychotic disorder per a structured clinical interview or if they met clinical criteria for alcohol, sedative/hypnotic, stimulant, cocaine, or marijuana dependence in the past 12 months, per the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). For participant safety, certain medical conditions were exclusionary, including current cancer, infection, inflammatory arthritis, or any uncontrolled medical problem. For tDCS safety, history of skull trauma, intracranial surgery, implanted hardware, or metal in the cranial cavity were exclusionary, as was the presence of broken skin or other dermal lesions where tDCS electrodes would be placed. As risks of tDCS to an unborn fetus are unknown, pregnant women were also excluded.
Three participants from PVAMC and one from Butler Hospital were ineligible after in-person screening. Five additional Butler Hospital participants withdrew or were lost to contact before the first experimental day. Thus, the final enrolled sample size was 21 participants (three female), 17 of whom were from PVAMC. For participants not referred by another clinician (e.g., self-referred via posted advertisements), we requested a release for medical records to verify a CLBP diagnosis. The Structured Clinical Interview for the DSM-IV-TR (SCID) Research Version, Patient Edition with Psychotic Screen, January 2007 revision [40], was used for the first nine participants, all of whom were tested at PVAMC. However, one PVAMC participant reported that the SCID triggered uncomfortable feelings relating to past trauma; after discussion among all investigators, it was agreed to replace the SCID with the shorter Mini International Neuropsychiatric Interview (M.I.N.I.) [41], version 5.0.0, which was also felt to be less burdensome; a prior version of the M.I.N.I. showed good concordance with a prior version of the SCID [42]. All participants at Butler Hospital were screened with the M.I.N.I.
Randomization
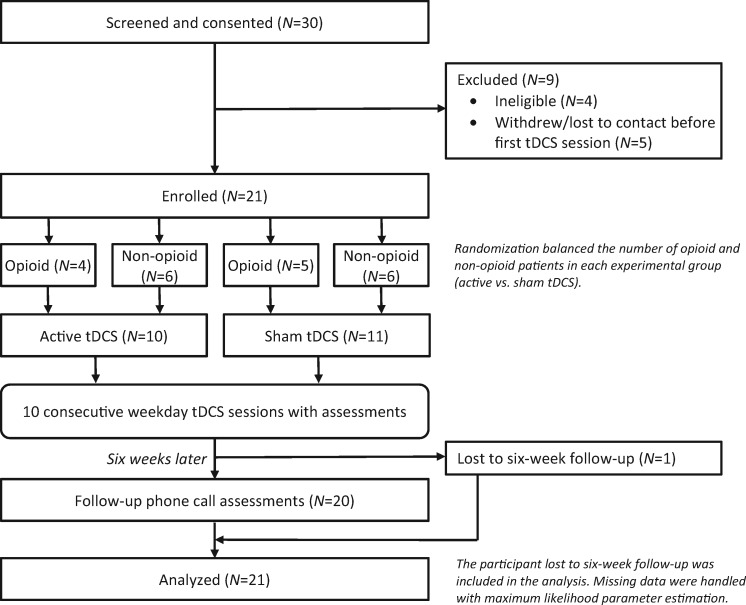
Participants (Table 1) were randomized in double-blinded fashion to receive 10 consecutive weekday sessions of either active tDCS or sham tDCS (placebo, more details below), one session per day (see Figure 1 for a study flowchart). Randomization was balanced (i.e., stratified) by prescription opioid status, so that both active and sham groups had roughly equal numbers of participants prescribed or not prescribed opioids, as illustrated in Table 2.
Table 1.
Baseline demographic data
| All Sample | Active tDCS | Sham tDCS | |
|---|---|---|---|
| Age, mean (SD), y | 63.1 (10.5) | 65.7 (8.8) | 60.7 (11.8) |
| Gender, No. (%) | |||
| Male | 18 (85.7) | 9 (90.0) | 9 (81.8) |
| Female | 3 (14.3) | 1 (10.0) | 2 (18.2) |
| Race and ethnicity, No. (%) | |||
| White | 14 (66.7) | 7 (70.0) | 7 (63.6) |
| Black or African American | 1 (4.8) | 0 (0.0) | 1 (9.1) |
| Hispanic or Latino | 1 (4.8) | 1 (10.0) | 0 (0) |
| American Indian or Alaska Native | 0 (0) | 0 (0) | 0 (0) |
| Asian | 0 (0) | 0 (0) | 0 (0) |
| Native Hawaiian or other Pacific Islander | 0 (0) | 0 (0) | 0 (0) |
| Other | 5 (23.8) | 2 (20.0) | 3 (27.3) |
| Years of education, mean (SD) | 14.4 (2.7) | 14.0 (2.6) | 14.7 (2.8) |
tDCS = transcranial direct current stimulation.
Figure 1.
Study flowchart. See also Table 2. Full details on the questionnaires and the timing appear in the Assessments section of the Methods. Further details on missing data and how they were handled appear in the Data Analysis section of the Methods. tDCS = transcranial direct current stimulation.
Table 2.
Participant allocation
| Opioid | No Opioid | Total | |
|---|---|---|---|
| Active | 4 | 6 | 10 |
| Sham | 5 | 6 | 11 |
| Total | 9 | 12 | 21 |
For Chronic Pain Acceptance Questionnaire, which was only obtained in 15 participants, the allocation was two active/opioid, four active/no opioid, four sham/opioid, five sham/no opioid.
tDCS
The tDCS was delivered with a battery-powered, microprocessor-controlled stimulator (DC-STIMULATOR PLUS, NeuroConn GmbH, Munich, Germany) with a coded study mode to facilitate randomization and blinding. We used a single-channel bipolar tDCS montage with 5 × 7 cm (35 cm2) carbon rubber electrodes enclosed in sponge pockets that were saturated in normal (0.9% NaCl) saline and held comfortably against the scalp with adjustable rubber straps. Stimulation intensity was 2 mA during active tDCS (average current density of 57 μ/cm2) for 20 minutes, plus 30 seconds of current “ramp up” and “ramp down” at the beginning and end of stimulation. We placed the cathodal electrode over FC1 on a 10–20 electroencephalography system, and an identical anodal return electrode was placed over the contralateral (right) mastoid process. This electrode montage for targeting the left dACC was adapted from our prior work [38] in healthy volunteers, intended to produce a current flow that would reduce activity in the left dACC.
Sham stimulation consisted of a 30-second current “ramp up” immediately followed by a 30-second “ramp down,” then 20 minutes of stimulation averaging no more than 0.002 mA (leakage current from the stimulator). This paradigm allows for participants to experience the tDCS-associated tingling sensation at the beginning of the session to improve the quality of the blind, with low expectation that the short duration of stimulation will appreciably modulate brain function.
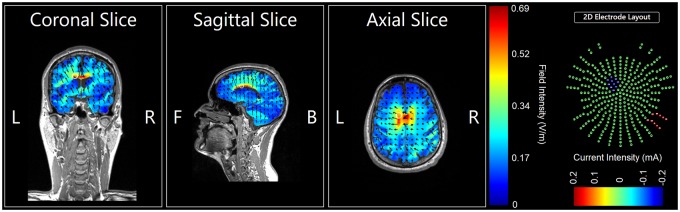
Electric Field Modeling
Although our electrode montage was empirically informed and adapted from prior work [38,43], for additional rigor, we performed post hoc confirmatory computer modeling to assess the montage’s ability to engage the dACC target. This electrical field modeling was performed with tDCS-Explore, version 4.0 (Soterix Medical, New York, NY, USA), which produces color-coded heat maps for qualitative assessment (Figure 2). The modeling demonstrated an electric field strength of >0.5 V/m in the left dACC, suggesting appreciable current flow through this deeper midline structure in participants.
Figure 2.
Electrode field model of study montage (coronal slice 133, sagittal slice 93, axial slice 77). The bipolar electrode montage for targeting the left dorsal anterior cingulate cortex (dACC) was adapted from our prior work [38]. Electrodes were 5 × 7 cm carbon rubber electrodes enclosed in sponge pockets saturated with normal (0.9% NaCl) saline, held comfortably in place on the scalp with adjustable rubber straps and connected to a battery-powered, microprocessor-controlled, single-channel stimulator (DC-STIMULATOR PLUS, NeuroConn GmbH, Munich, Germany). The cathodal electrode was placed at FC1 on the 10–20 electroencephalography system, and the anodal return electrode was placed over the contralateral (right) mastoid. Although this montage was empirically informed, for additional rigor, we performed post hoc confirmatory computer modeling to assess the montage’s ability to engage the dACC target. This modeling was performed with tDCS-Explore, v. 4.0 (Soterix Medical, New York, NY, USA), which produced the color-coded heat maps shown here for qualitative assessment. The white circles indicate the approximate location of the left dACC. The modeling demonstrated an electric field strength of >0.5 V/m at the left dACC. For a full color figure, please see the online version of this article.
Assessments
Participants’ pain intensity was measured with the 11-point DVPRS [39] with a possible range of 0–10 (10 is highest pain intensity). Perceived pain interference in daily functioning was measured by the West Haven-Yale Multidimensional Pain Inventory’s General Activity Subscale (WHY-MPI-C) [44], with 18 items summed to give a score ranging from 0 to 108 (higher scores indicate more activity). The widely used Roland Morris Disability Questionnaire (RMDQ) measured back pain–specific disability [45,46], with a possible score ranging from 0 to 24 (higher scores indicate greater pain disability). Pain acceptance was measured with the Chronic Pain Acceptance Questionnaire (CPAQ-8), a validated measure of the ability to experience ongoing pain but still engage in enjoyable activities not focused on avoiding or reducing pain [47,48]; scores could range from 0 to 48 (higher scores indicate greater pain acceptance). The Pain Anxiety Symptoms Scale (PASS-20) measured pain-related fear and avoidance [49]; scores could range from 0 to 100 (higher scores indicate greater pain anxiety). Depression and anxiety symptoms were assessed with the nine-item Patient Health Questionnaire (PHQ-9; score range 0–27, higher is more depressed) [50,51] and seven-item Generalized Anxiety Disorder scale (GAD-7; score range 0–21, higher is more anxious) [52,53]. We also tracked participants’ treatment expectations and satisfaction with the Credibility/Expectancy Questionnaire (CEQ) and Client Satisfaction Questionnaire-8 (CSQ-8; score range 8–32, higher is greater satisfaction), respectively [54–56]. Because the CEQ employs both percent and nine-point integer ratings for different items, we followed what has been done previously in CLBP studies [57] and linearly transformed the percent ratings to integer ratings. We then summed the scores for the six CEQ items to obtain a total score, which could range from 6 to 54 (higher is greater credibility and expectancy).
All assessments except for CSQ-8 were administered in person at the start of an experimental session before commencing tDCS; CSQ-8 was administered immediately after tDCS finished. Day 1 assessment scores were therefore considered baseline values for the purposes of this study. The follow-up assessment was scheduled to be conducted via telephone six weeks after the final tDCS session (i.e., eight weeks after the first tDCS session) and involved no tDCS. DVPRS was obtained each session and at six-week follow-up. WHY-MPI-C, RMDQ, CPAQ-8, PASS-20, PHQ-9, and GAD-7 were obtained on day 1, day 5, day 10, and at six-week follow-up. CEQ and CSQ-8 were obtained on day 1 and day 10. For a full schedule of assessments, see the Supplementary Data.
Data Analysis
Multilevel mixed-effects linear regression models were used to analyze the complete data set, at time points of day 1 (baseline), day 5, day 10, and six-week follow-up. An intention-to-treat approach was taken. Statistical significance was defined as a two-tailed P value of ≤0.05. Regression models and analyses were performed with Stata 14.2 (StataCorp LLC, College Station, TX, USA). Descriptive statistics (second and fourth columns of Table 3) were computed with R 3.3.2 (http://www.r-project.org/).
Table 3.
Descriptive statistics (second and fourth columns) and multiple linear regression modeling results (sixth column) showing changes in outcome variables as a function of stimulation (active vs sham tDCS) over time
| Score and No., Mean (SD) [Range] |
P | ||||
|---|---|---|---|---|---|
| Active tDCS | No. | Sham tDCS | No. | ||
| DVPRS | |||||
| Day 1 | 5.4 (2.2) [1–9] | 10 | 5.5 (1.1) [4–7] | 11 | 0.97 |
| Day 5 | 4.8 (2.7) [0–8] | 10 | 4.6 (2.3) [1–8] | 11 | 0.82 |
| Day 10 | 4.0 (2.4) [0–8] | 10 | 4.0 (2.2) [1–8] | 11 | 0.91 |
| 6-wk follow-up | 4.3 (2.5) [0–9] | 10 | 4.8 (1.8) [2–8] | 10 | 0.58 |
| WHY-MPI-C | |||||
| Day 1 | 31.3 (16.1) [9–53] | 10 | 36.9 (13.3) [14–60] | 11 | 0.45 |
| Day 5 | 39.9 (23.0) [9–85] | 10 | 40.8 (18.9) [8–82] | 11 | 0.41 |
| Day 10 | 41.6 (21.9) [12–78] | 10 | 41.0 (15.8) [15–68] | 11 | 0.26 |
| 6-wk follow-up | 48.4 (21.2) [15–85] | 10 | 35.3 (17.7) [11–73] | 10 | 0.002* |
| RMDQ | |||||
| Day 1 | 13.8 (5.4) [5–20] | 10 | 12.5 (4.9) [3–19] | 11 | 0.46 |
| Day 5 | 13.2 (6.0) [1–20] | 10 | 12.5 (5.3) [2–21] | 11 | 0.54 |
| Day 10 | 12.5 (5.6) [0–19] | 10 | 12.8 (4.1) [4–18] | 11 | 0.13 |
| 6-wk follow-up | 12.7 (5.6) [1–20] | 10 | 15.4 (5.5) [3–22] | 10 | 0.001* |
| CPAQ-8 | |||||
| Day 1 | 31.0 (7.2) [22–42] | 6 | 29.9 (10.5) [10–48] | 9 | 0.98 |
| Day 5 | 29.8 (5.2) [22–36] | 5 | 27.9 (5.6) [15–35] | 9 | 0.52 |
| Day 10 | 30.5 (4.6) [23–36] | 6 | 28.6 (8.4) [14–45] | 9 | 0.74 |
| 6-wk follow-up | 32.5 (5.5) [26–42] | 6 | 27.9 (8.6) [14–39] | 8 | 0.40 |
| PHQ-9 | |||||
| Day 1 | 11.0 (7.8) [3–22] | 10 | 8.1 (5.2) [0–14] | 10 | 0.27 |
| Day 5 | 10.0 (8.3) [0–22] | 10 | 7.0 (4.7) [0–14] | 11 | 0.89 |
| Day 10 | 8.9 (7.1) [2–20] | 10 | 6.8 (4.9) [0–15] | 11 | 0.65 |
| 6-wk follow-up | 7.4 (6.1) [1–17] | 10 | 8.9 (6.0) [0–17] | 10 | 0.003* |
| GAD-7 | |||||
| Day 1 | 7.5 (7.6) [0–19] | 10 | 8.3 (5.2) [0–14] | 11 | 0.77 |
| Day 5 | 7.2 (7.4) [0–18] | 10 | 6.2 (5.9) [0–18] | 11 | 0.31 |
| Day 10 | 6.2 (6.7) [0–18] | 10 | 7.0 (6.3) [0–17] | 11 | 0.96 |
| 6-wk follow-up | 6.0 (6.6) [0–17] | 10 | 4.9 (4.5) [0–13] | 10 | 0.32 |
| PASS-20 | |||||
| Day 1 | 34.4 (22.8) [6–80] | 10 | 37.5 (25.3) [4–74] | 11 | 0.75 |
| Day 5 | 34.9 (23.5) [2–78] | 10 | 30.4 (21.2) [2–71] | 11 | 0.27 |
| Day 10 | 27.8 (24.2) [0–72] | 9 | 27.4 (21.3) [1–58] | 11 | 0.67 |
| 6-wk follow-up | 26.3 (23.6) [0–59] | 10 | 26.4 (22.0) [1–71] | 10 | 0.67 |
| CEQ | |||||
| Day 1 | 35.9 (7.8) [25.2–51.6] | 10 | 38.5 (7.6) [29.6–53.2] | 11 | 0.49 |
| Day 10 | 38.8 (9.7) [23.6–54.0] | 9 | 33.7 (9.0) [22.0–51.6] | 10 | 0.038* |
| CSQ-8 | |||||
| Day 1 | 29.5 (3.3) [21–32] | 10 | 29.2 (2.4) [25–32] | 11 | 0.83 |
| Day 10 | 29.3 (2.2) [25–32] | 10 | 28.3 (2.9) [24–32] | 11 | 0.57 |
All assessments except for CSQ-8 were administered in person at the start of an experimental session before commencing tDCS; CSQ-8 was administered immediately after tDCS finished. Day 1 assessment scores were therefore considered baseline values for the purposes of this study. Statistical significance was defined as the traditional two-tailed P ≤ 0.05. WHY-MPI-C General Activity Subscale, RMDQ, PHQ-9, and CEQ all showed significant improvements at the six-week follow-up telephone assessment, which occurred on average 6.2 weeks after the day 10 tDCS session (tDCS sessions occurred only on weekdays); one PVAMC participant was lost to 6-week follow-up. The third and fifth columns report the No. of participants included in the model for a particular condition and measure at a given time point. Differences from the last column of Table 2 are due to CPAQ-8 not being obtained in all participants, the participant lost to follow-up, and missed/incomplete measures from other participants. See the Methods for how missing data were handled. CEQ is provided as a total score that could range from 6 to 54; see the text for full details of how this was calculated. For simplicity, DVPRS is reported only for the following time points: day 1, day 5, day 10, and 6-week follow-up.
CEQ = Credibility/Expectancy Questionnaire; CPAQ-8 = Chronic Pain Acceptance Questionnaire; CSQ-8 = Client Satisfaction Questionnaire-8; DVPRS = Defense and Veterans Pain Rating Scale; GAD-7 = Generalized Anxiety Disorder scale; PASS-20 = Pain Anxiety Symptoms Scale; PHQ-9 = Patient Health Questionnaire; RMDQ = Roland Morris Disability Questionnaire; tDCS = transcranial direct current stimulation; WHY-MPI-C = West Haven-Yale Multidimensional Pain Inventory’s General Activity Subscale.
Maximum likelihood parameter estimation was used [58] to handle missing data under the “missing at random” assumption for all variables except CPAQ-8. For CPAQ-8—added to the study after it was underway—missing data were handled under the “missing completely at random” assumption, with analysis for this variable performed on the reduced sample of complete CPAQ-8 data.
Results
Participants
Out of 30 participants (seven female) consented, a total of 21 (three female) were enrolled and completed all 10 tDCS sessions and associated assessments (Table 2): 17 at PVAMC and four (two female) at Butler Hospital (overall, nine participants were ineligible, withdrew, or were lost to contact before commencing tDCS). CPAQ-8 assessments were obtained in 15 participants. One Butler Hospital participant received a steroid back injection five days before the follow-up phone call; this participant was asked to provide retrospective assessment scores for the day before receiving the steroid injection (5.6 weeks after tDCS day 10). One PVAMC participant was lost to follow-up; for the remaining 20 participants, the average time to follow-up was 6.2 weeks.
Outcomes
We first examined for effects of active vs sham tDCS over time (interaction terms), for time points day 1, day 5, day 10, and six-week follow-up (i.e., eighth week of the study overall). Comparing active with sham tDCS (Table 3), DVPRS scores did not significantly change (all |z| < 0.56, all P > 0.58). WHY-MPI-C scores were significantly increased at six-week follow-up (z = 3.11, P = 0.002) only (all other |z| < 1.13, all other P > 0.26). RMDQ scores were significantly reduced at six-week follow-up (z = –3.23, P = 0.001) only (all other time points |z| < 1.51, P > 0.13). CPAQ-8 scores did not significantly change (all |z| < 0.86, all P > 0.39). PASS-20 scores also did not significantly change (all |z| < 1.13, all P > 0.26), nor did GAD-7 scores (all |z| < 1.02, all P > 0.31). PHQ-9 scores were significantly reduced at six-week follow-up (z = –2.96, P = 0.003) only (all other time points |z| < 1.11, P > 0.27). CEQ scores were significantly increased at day 10 (z = 2.08, P = 0.038; day 1 z = –0.70, P = 0.49), but CSQ-8 scores did not significantly change (all |z| < 0.58, all P > 0.56).
Regarding comparisons of opioid and nonopioid participants over time (irrespective of tDCS condition), day 1 (baseline) CPAQ-8 was significantly lower for participants prescribed opioids (z = –3.59, P < 0.001). There were no other significant differences between opioid and nonopioid participants at any time point (WHY-MPI-C six-week follow-up |z| = 1.74, P = 0.08; PHQ-9 six-week follow-up |z| = 1.68, P = 0.09; all other |z| < 1.56, all other P > 0.12).
We also performed separate omnibus tests to detect any overall effect of active (vs sham) tDCS. Collapsing across all time points did not detect a significant effect (all Χ2 < 3.49, all P > 0.06). However, collapsing across time points day 5, day 10, and six-week follow-up (i.e., excluding the baseline values of day 1), active tDCS was significantly different than sham for WHY-MPI-C scores (Χ2 = 4.34, P = 0.037), RMDQ (Χ2 = 4.84, P = 0.028), and CEQ (Χ2 = 4.31, P = 0.038). Note that because CEQ was only collected on days 1 and 10, this last omnibus test is equivalent to the multiple linear regression result above.
Discussion
To the authors’ knowledge, this is the first double-blinded RCT of multiple tDCS sessions targeting dACC and delivered to a clinical population with CLBP. Participants who received active tDCS exhibited significantly reduced pain interference, pain disability, and depression symptoms at six-week follow-up. The multisession tDCS paradigm was well tolerated; no participants withdrew from the study after commencing tDCS, and all but one participant completed the six-week follow-up assessment. Satisfaction rates were high regardless of treatment group (day 5 and day 10 mean CSQ-8 scores all exceeded 28 out of a possible 32 points). Participants were also well engaged; treatment expectations (CEQ) significantly increased by the tenth tDCS treatment day in the active group.
Critically, our randomization scheme successfully balanced participants by opioid status (Table 2). Only CPAQ-8 exhibited a statistically significant baseline difference (day 1, assessed before tDCS), likely reflecting the measure being obtained in only 15 of 21 participants, with poorer group balancing for this outcome.
Despite a small (N = 21) sample size that substantially limited statistical power to detect subtle tDCS-driven effects, significant time-dependent improvements were noted at six-week follow-up for pain-related interference in daily functioning (WHY-MPI-C), disability specifically related to back pain (RMDQ), and depression symptoms (PHQ-9). The omnibus tests further suggest that the improvements in daily functioning, disability, and depression were unlikely to have been due merely to baseline differences in the groups, but were due to actual change over time. Recently, investigators have noted that tDCS effects accumulate over time and may take several weeks to manifest fully; thus, no significant change may be observed during an acute treatment phase [59,60]. Our results are entirely consistent with this observation. Furthermore, such delayed effects are common with psychiatric treatments. Indeed, both pharmacologic (e.g., selective serotonin or selective serotonin-norepinephrine reuptake inhibitors) and nonpharmacologic (e.g., neuromodulation with electroconvulsive therapy or transcranial magnetic stimulation) modalities for treating depression can take weeks to demonstrate efficacy.
The noted improvements in pain interference, daily functioning, and back pain disability suggest that these more functionally oriented and pain-specific outcomes may be preferentially improved in CLBP patients receiving tDCS targeting the dACC per our montage. Although our results suggest that such improvements may be modest, they should be considered in the context of the burgeoning opioid crisis and the pressing need for effective nonpharmacologic pain treatments, even if resulting gains are incremental. The improvement in self-reported general depression could suggest modulation of the affective component of pain, a hypothesis that should be tested in better-powered RCTs. These improvements correlated with increases in treatment expectancy and credibility (CEQ), suggesting that this parameter may be modifiable in a manner predictive of an improved outcome [57]. However, given our results and the recognition that pain has equally important sensory and affective components [3], future studies should specifically assess pain-related mood symptoms as a primary outcome. Given the small size of our study, these results should be interpreted cautiously and used to generate hypotheses to be tested in future larger, purpose-designed studies.
Based on our prior work [38], we expected that tDCS targeting the dACC would improve pain tolerability and pain-related affective symptoms without necessarily changing pain intensity as measured by the DVPRS. The present results are consistent with this expectation, as tDCS group (active vs sham) had no effect on daily DVPRS pain intensity assessments; future larger studies will be necessary to test this potential dissociation more thoroughly.
The lack of significant change over time in either pain-related or general anxiety (PASS-20, GAD) could suggest that this symptom dimension is less involved in CLBP-related disability than mood symptoms. Certain chronic pain conditions such as fibromyalgia have as key features anxiety symptoms such as catastrophizing [14], but this may be less significant in CLBP. Moreover, in the present study, we did not rate or stratify participants by premorbid anxiety levels or degree of catastrophizing. Further study is needed before any definitive pronouncements can be made on the possible effects of tDCS on anxiety in CLBP.
Weaknesses
This study has several weaknesses that future work should address. As already mentioned, the small sample size limited statistical power. Post hoc modeling suggested that our electrode montage produced appreciable current flow through the left dACC, a fairly deep midline structure (with the caveat that individual differences in brain morphology cannot be accounted for). Although post hoc electric field modeling suggested that our electrode montage, empirically informed by prior work [38], likely produced current flow through the left dACC, we cannot guarantee that the montage optimally targeted the left dACC in all participants. A more rigorous approach to demonstrating target engagement would be utilizing iterative models to optimize the electrode montage for maximal expected current flow in the desired direction through the intended cortical target before commencing the study; this would be especially important if a different cortical target or a different size or number of electrodes was envisioned. However, over-reliance on such computer models is inadvisable due to the lack of empirical validation [32]. Functional neuroimaging and functional connectivity analysis before and after a series of tDCS sessions would be superior in their ability to provide empirical evidence of target engagement, but the present study unfortunately lacked the resources to support such an approach.
Due to the limitations of bipolar (two-electrode) tDCS equipment providing only one channel of stimulation and our choice to follow prior work [38], we were not able to address laterality effects of pain perception or processing. Indeed, there is evidence of lateralization to the right hemisphere and right dACC [61–64], suggesting that our decision to target the left dACC was suboptimal. However, due to tDCS’s aforementioned lack of focality, the electric field modeling suggests appreciable current flow through the right dACC with the FC1-right mastoid electrode montage that we used (Figure 2). Therefore, an intriguing unanswered question is whether an FC2-left mastoid montage may have produced greater effects.
The tDCS experimental paradigm and equipment give rise to additional limitations. Sham is imperfect. Despite a brief ramp-up and ramp-down at the beginning of a sham stimulation session to simulate the tingling associated with active stimulation, at the 2-mA current amplitude used in the current study, blinding may not be complete [65,66]. Furthermore, the NeuroConn DC-STIMULATOR PLUS produces an approximately 0.002-mA leakage current during sham stimulation. Although this is three orders of magnitude lower than the active stimulation amplitude, one must not dismiss that multiple extended, low-intensity periods of tDCS may have some neuromodulatory effect [67]. Furthermore, the tDCS operator must be careful not to view the impedance readout or charge state of the battery during or after a stimulation session as doing so might inadvertently compromise the blind. Even so, the presence of the skin erythema that occurs with active stimulation may compromise the blind [31,65].
Finally, tDCS is nonfocal. A general rule of thumb with electromagnetic forms of NIBS is that they sacrifice focality for depth, which is particularly apparent with the bipolar (or one-channel) tDCS approach used in the present study (Figure 2). Significant current flow is expected through structures adjacent to or overlying the intended cortical target, and this is a particular confound when considering deep midline structures such as the dACC. Variations in participants’ individual anatomy may also lead to localized differences in current intensity and direction within the cortex. “High-definition” tDCS has been developed to improve the focality of the stimulation, typically by using arrays of up to 64 smaller electrodes—backed by computer modeling and simultaneous electroencephalography recording—to improve spatial resolution at deeper targets including the dACC [68]. Such approaches must also account for the direction of induced current flow relative to target structures given their neuronal organization [69]. Emerging noninvasive approaches that do not exert their direct effects via electromagnetic energy have shown intriguing potential for both precision and depth of stimulation; for example, transcranial ultrasound, which can use low-intensity acoustic energy to stimulate neural tissue nondestructively [70,71], may improve mood in chronic pain patients [72].
Future Work
In addition to addressing the above weaknesses, future studies using NIBS protocols to modulate the affective component of CLBP can be further improved. For simplicity, the present study did not extensively explore the parameter space for stimulation. Stimulation duration and total number of treatment sessions are key variables that should be systematically explored, along with frequency of treatments (e.g., daily, twice daily, every other day, etc.). Recent evidence suggests that tDCS has cumulative effects [59,60] on cortical plasticity that would benefit from an increased number of sessions (more than the 10 used in this study) and longer periods of follow-up (beyond the relatively short-term six weeks we used). However, because the brain is a dynamic and nonlinear system in which tDCS-associated responses are likely state-dependent, it would be an oversimplification to rely on a “dose-response” conceptualization of tDCS effects [73]. From a safety standpoint, a recent systematic review did indicate that increasing tDCS exposure (e.g., number of sessions) does not seem to increase risk of adverse events [74].
Cingulate laterality effects and additional cortical targets representing nodes in the pain processing network should also be explored, ideally with higher-focality neuromodulation approaches informed by electrode field modeling and confirmed by real-time neuroimaging. The ability of transcranial alternating current stimulation (tACS) to enhance or entrain cortical oscillations [31,75] further expands the parameter space for stimulation–by permitting adjustment of waveform and frequency of stimulation (including repetitive burst-style stimulation)–to parameters that by definition are not present with tDCS.
Finally, because tDCS is by definition a subthreshold neuromodulation scheme, pairing it with a desired behavior may increase efficacy [31,32]. Thus, future studies employing multimodal approaches may benefit from combining tDCS with a CBT program tailored to pain-related symptoms, especially for lesser-studied forms of persistent pain such as subacute low back pain [76]. In this case, careful consideration would need to be given to the optimal timing of tDCS relative to the CBT intervention.
Conclusions
We conducted a double-blinded placebo-controlled RCT of repeated sessions of tDCS targeting the left dACC with the intention of inhibiting the region to modulate the affective component of CLBP. Study retention was high; all enrolled participants completed all 10 tDCS sessions, and only one was lost to six-week follow-up. Our results suggest that participants who received active tDCS with cathodal electrode targeting the left dACC (and a contralateral mastoid anodal return electrode) may show improvements in pain-related functioning and distress (WHY-MPI-C and RMDQ), as well as in depression (PHQ-9), after the acute treatment phase. These improvements correlated with increases in treatment expectancy and credibility (CEQ). However, our study’s small sample size limited statistical power. Thus, larger, better-powered double-blinded RCTs are needed before definitive conclusions can be drawn. Future studies should also explore, in a systematic manner, the parameter space of tDCS as applied to modulate complex cortical processes. They should consider additional cortical targets, high-density multi-electrode arrays for better spatial resolution, and neuroimaging (instead of the electrode field modeling done in our study) to demonstrate engagement of desired cortical targets. Finally, as subthreshold neuromodulation with tDCS may be more effective when paired with another intervention, a multimodal approach that combines, for example, tDCS with pain-focused CBT may also be prudent.
Supplementary Material
Acknowledgments
We thank Sharon Longo, Ganaelle Joseph-Senatus, Evalina Bond, and Harrison Burgess, PharmD, for assistance in screening and testing of study participants. We thank Brittney Blanchette for assistance in preparing study materials for publication.
Supplementary Data
Supplementary data are available at Pain Medicine online.
Funding sources: This work was supported by internal funding from Butler Hospital, the National Institute of Mental Health R25 MH101076 (TYM), a 2015 NARSAD Young Investigator Grant (TYM), the Brown Institute for Brain Science, and the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service and the Center of Excellence for Neurorestoration and Neurotechnology at the Providence VA Medical Center.
Disclaimer: The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Conflicts of interest: TYM has consulted for Janssen Pharmaceuticals, Inc. and Ad Scientiam SAS, both unrelated to the present work. The authors report no other potential conflicts.
References
- 1. Manchikanti L, Singh V, Datta S, Cohen SP, Hirsch JA; American Society of Interventional Pain Physicians. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician 2009;12(4):E35–70. [PubMed] [Google Scholar]
- 2. Goertz M, Thorson D, Bonsell J, et al. Adult Acute and Subacute Low Back Pain. Report No. NGC: 009520. Bloomington, MN: Institute for Clinical Systems Improvement; 2012. Available at: https://www.sunshinehealth.com/content/dam/centene/Sunshine/pdfs/1_lbp.pdf. (accessed October 2018).
- 3. IASP. IASP terminology. 2017. Available at: http://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698 (accessed August 2018).
- 4. Crombez G, Eccleston C, Van Damme S, Vlaeyen JWS, Karoly P.. Fear-avoidance model of chronic pain: The next generation. Clin J Pain 2012;28(6):475–83. [DOI] [PubMed] [Google Scholar]
- 5. Tang NKY, Crane C.. Suicidality in chronic pain: A review of the prevalence, risk factors and psychological links. Psychol Med 2006;36(5):575–86. [DOI] [PubMed] [Google Scholar]
- 6. Mansour AR, Baliki MN, Huang L, et al. Brain white matter structural properties predict transition to chronic pain. Pain 2013;154(10):2160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loggia ML, Kim J, Gollub RL, et al. Default mode network connectivity encodes clinical pain: An arterial spin labeling study. Pain 2013;154(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baliki MN, Petre B, Torbey S, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 2012;15(8):1117–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: The SPACE randomized clinical trial. JAMA 2018;319(9):872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wachholtz A, Foster S, Cheatle M.. Psychophysiology of pain and opioid use: Implications for managing pain in patients with an opioid use disorder. Drug Alcohol Depend 2015;146:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eisenberg E, Suzan E, Pud D.. Opioid-induced hyperalgesia (OIH): A real clinical problem or just an experimental phenomenon? J Pain Symptom Manage 2015;49(3):632–6. [DOI] [PubMed] [Google Scholar]
- 12. Jamison RN, Edwards RR.. Risk factor assessment for problematic use of opioids for chronic pain. Clin Neuropsychol 2013;27(1):60–80. [DOI] [PubMed] [Google Scholar]
- 13. Reid MC, Otis J, Barry LC, Kerns RD.. Cognitive-behavioral therapy for chronic low back pain in older persons: A preliminary study. Pain Med Malden Mass 2003;4(3):223–30. [DOI] [PubMed] [Google Scholar]
- 14. Lazaridou A, Kim J, Cahalan CM, et al. Effects of cognitive-behavioral therapy (CBT) on brain connectivity supporting catastrophizing in fibromyalgia. Clin J Pain 2017;33(3):215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kerns RD, Sellinger J, Goodin BR.. Psychological treatment of chronic pain. Annu Rev Clin Psychol 2011;7:411–34. [DOI] [PubMed] [Google Scholar]
- 16. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC.. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull 2007;133(4):581–624. [DOI] [PubMed] [Google Scholar]
- 17. Cunningham NR, Kashikar-Zuck S.. Nonpharmacologic treatment of pain in rheumatic diseases and other musculoskeletal pain conditions. Curr Rheumatol Rep 2013;15(2):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jamison RN. Learning to Master Your Chronic Pain. Sarasota, FL: Professional Resource Press; 1996. [Google Scholar]
- 19. Mylius V, Borckardt JJ, Lefaucheur J-P.. Noninvasive cortical modulation of experimental pain. Pain 2012;153(7):1350–63. [DOI] [PubMed] [Google Scholar]
- 20. Ploner M, Gross J, Timmermann L, Schnitzler A.. Cortical representation of first and second pain sensation in humans. Proc Natl Acad Sci U S A 2002;99(19):12444–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhuo M. Long-term potentiation in the anterior cingulate cortex and chronic pain. Philos Trans R Soc Lond B Biol Sci 2014;369(1633):20130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vogt BA, Wiley RG, Jensen EL.. Localization of Mu and delta opioid receptors to anterior cingulate afferents and projection neurons and input/output model of Mu regulation. Exp Neurol 1995;135(2):83–92. [DOI] [PubMed] [Google Scholar]
- 23. Spangler WJ, Cosgrove GR, Ballantine HT, et al. Magnetic resonance image-guided stereotactic cingulotomy for intractable psychiatric disease. Neurosurgery 1996;38(6):1071–6; discussion 1076–8. [PubMed] [Google Scholar]
- 24. Foltz EL, White LE.. The role of rostral cingulumotomy in “pain” relief. Int J Neurol 1968;6(3–4):353–73. [PubMed] [Google Scholar]
- 25. Foltz EL, White LE.. Pain “relief” by frontal cingulumotomy. J Neurosurg 1962;19:89–100. [DOI] [PubMed] [Google Scholar]
- 26. Ballantine HT, Cassidy WL, Flanagan NB, Marino R.. Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg 1967;26(5):488–95. [DOI] [PubMed] [Google Scholar]
- 27. Aghion DM, Cosgrove GR. Surgical Interventions for Pain. 2014. Available at: https://www.growkudos.com/publications/10.1016%252Fb978-0-12-398389-3.00005-4 (accessed December 2017).
- 28. Boccard SGJ, Fitzgerald JJ, Pereira EAC, et al. Targeting the affective component of chronic pain: A case series of deep brain stimulation of the anterior cingulate cortex. Neurosurgery 2014;74(6):628–37. [DOI] [PubMed] [Google Scholar]
- 29. Boccard SGJ, Prangnell SJ, Pycroft L, et al. Long-term results of deep brain stimulation of the anterior cingulate cortex for neuropathic pain. World Neurosurg 2017;106:625–37. [DOI] [PubMed] [Google Scholar]
- 30. Rahman A, Reato D, Arlotti M, et al. Cellular effects of acute direct current stimulation: Somatic and synaptic terminal effects. J Physiol 2013;591(10):2563–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Woods AJ, Antal A, Bikson M, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol off J Int Fed Clin Neurophysiol 2016;127(2):1031–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Philip NS, Nelson BG, Frohlich F, et al. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry 2017;174(7):628–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Valle A, Roizenblatt S, Botte S, et al. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: Results of a randomized, sham-controlled longitudinal clinical trial. J Pain Manag 2009;2(3):353–61. [PMC free article] [PubMed] [Google Scholar]
- 34. Khedr EM, Omran EAH, Ismail NM, et al. Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: A double blinded, randomized clinical trial. Brain Stimulat 2017;10(5):893–901. [DOI] [PubMed] [Google Scholar]
- 35. Fregni F, Boggio PS, Lima MC, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain 2006;122(1–2):197–209. [DOI] [PubMed] [Google Scholar]
- 36. Antal A, Terney D, Kühnl S, Paulus W.. Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition. J Pain Symptom Manage 2010;39(5):890–903. [DOI] [PubMed] [Google Scholar]
- 37. Ahn H, Woods AJ, Kunik ME, et al. Efficacy of transcranial direct current stimulation over primary motor cortex (anode) and contralateral supraorbital area (cathode) on clinical pain severity and mobility performance in persons with knee osteoarthritis: An experimenter- and participant-blinded, randomized, sham-controlled pilot clinical study. Brain Stimulat 2017;10(5):902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mariano TY, van't Wout M, Jacobson BL, et al. Effects of transcranial direct current stimulation (tDCS) on pain distress tolerance: A preliminary study. Pain Med 2015;16(8):1580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buckenmaier CC, Galloway KT, Polomano RC, et al. Preliminary validation of the Defense and Veterans Pain Rating Scale (DVPRS) in a military population. Pain Med 2013;14(1):110–23. [DOI] [PubMed] [Google Scholar]
- 40. First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders—Patient Edition with Psychotic Screen. SCID-I/P W/PSYCHOTIC SCREEN, 1/2007 Revision. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 2007. Available at: http://www.scid4.org/info/refscid.html.
- 41. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(suppl 20):22–33; quiz 34–57. [PubMed] [Google Scholar]
- 42. Sheehan DV, Sheehan KH, Shytle RD, et al. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). J Clin Psychiatry 2010;71(3):313–26. [DOI] [PubMed] [Google Scholar]
- 43. Mariano TY, Van’t Wout M, Garnaat SL, Rasmussen SA, Greenberg BD.. Transcranial direct current stimulation (tDCS) targeting left dorsolateral prefrontal cortex modulates task-induced acute pain in healthy volunteers. Pain Med 2016;17(4):737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kerns RD, Turk DC, Rudy TE.. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain 1985;23(4):345–56. [DOI] [PubMed] [Google Scholar]
- 45. Roland M, Morris R.. A study of the natural history of low-back pain. Part II: Development of guidelines for trials of treatment in primary care. Spine 1983;8(2):145–50. [DOI] [PubMed] [Google Scholar]
- 46. Roland M, Fairbank J.. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine 2000;25(24):3115–24. [DOI] [PubMed] [Google Scholar]
- 47. Rovner GS, Arestedt K, Gerdle B, Börsbo B, McCracken LM.. Psychometric properties of the 8-item Chronic Pain Acceptance Questionnaire (CPAQ-8) in a Swedish chronic pain cohort. J Rehabil Med 2014;46(1):73–80. [DOI] [PubMed] [Google Scholar]
- 48. Fish RA, McGuire B, Hogan M, Morrison TG, Stewart I.. Validation of the Chronic Pain Acceptance Questionnaire (CPAQ) in an Internet sample and development and preliminary validation of the CPAQ-8. Pain 2010;149(3):435–43. [DOI] [PubMed] [Google Scholar]
- 49. McCracken LM, Dhingra L.. A short version of the Pain Anxiety Symptoms Scale (PASS-20): Preliminary development and validity. Pain Res Manag 2002;7(1):45–50. [DOI] [PubMed] [Google Scholar]
- 50. Kroenke K, Spitzer RL, Williams JB.. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Spitzer RL, Kroenke K, Williams JB.. Validation utility of a self-report version of PRIME-MD: The PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. JAMA 1999;282(18):1737–44. [DOI] [PubMed] [Google Scholar]
- 52. Löwe B, Decker O, Müller S, et al. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care 2008;46(3):266–74. [DOI] [PubMed] [Google Scholar]
- 53. Spitzer RL, Kroenke K, Williams JBW, Löwe B.. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med 2006;166(10):1092–7. [DOI] [PubMed] [Google Scholar]
- 54. Nguyen TD, Attkisson CC, Stegner BL.. Assessment of patient satisfaction: Development and refinement of a service evaluation questionnaire. Eval Program Plann 1983;6(3–4):299–313. [DOI] [PubMed] [Google Scholar]
- 55. Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD.. Assessment of client/patient satisfaction: Development of a general scale. Eval Program Plann 1979;2(3):197–207. [DOI] [PubMed] [Google Scholar]
- 56. Devilly GJ, Borkovec TD.. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry 2000;31(2):73–86. [DOI] [PubMed] [Google Scholar]
- 57. Smeets RJEM, Beelen S, Goossens MEJB, et al. Treatment expectancy and credibility are associated with the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. Clin J Pain 2008;24(4):305–15. [DOI] [PubMed] [Google Scholar]
- 58. Ware JH, Harrington D, Hunter DJ, D’Agostino RB.. Missing data. N Engl J Med 2012;367(14):1353–4. [Google Scholar]
- 59. Sampaio-Junior B, Tortella G, Borrione L, et al. Efficacy and safety of transcranial direct current stimulation as an add-on treatment for bipolar depression. A randomized clinical trial. JAMA Psychiatry 2018;75(2):158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bikson M, Brunoni AR, Charvet LE, et al. Rigor and reproducibility in research with transcranial electrical stimulation: An NIMH-sponsored workshop. Brain Stimulat 2018;11(3):465–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Duerden EG, Albanese M-C.. Localization of pain-related brain activation: A meta-analysis of neuroimaging data. Hum Brain Mapp 2013;34(1):109–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hsieh JC, Stone-Elander S, Ingvar M.. Anticipatory coping of pain expressed in the human anterior cingulate cortex: A positron emission tomography study. Neurosci Lett 1999;262(1):61–4. [DOI] [PubMed] [Google Scholar]
- 63. Kobayashi Y, Kurata J, Sekiguchi M, et al. Augmented cerebral activation by lumbar mechanical stimulus in chronic low back pain patients: An FMRI study. Spine 2009;34(22):2431–6. [DOI] [PubMed] [Google Scholar]
- 64. Symonds LL, Gordon NS, Bixby JC, Mande MM.. Right-lateralized pain processing in the human cortex: An FMRI study. J Neurophysiol 2006;95(6):3823–30. [DOI] [PubMed] [Google Scholar]
- 65. Palm U, Reisinger E, Keeser D, et al. Evaluation of sham transcranial direct current stimulation for randomized, placebo-controlled clinical trials. Brain Stimulat 2013;6(4):690–5. [DOI] [PubMed] [Google Scholar]
- 66. O’Connell NE, Cossar J, Marston L, et al. Rethinking clinical trials of transcranial direct current stimulation: Participant and assessor blinding is inadequate at intensities of 2mA. PLoS One 2012;7(10):e47514.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Loo CK, Husain MM, McDonald WM, et al. International randomized-controlled trial of transcranial direct current stimulation in depression. Brain Stimulat 2018;11(1):125–33. [DOI] [PubMed] [Google Scholar]
- 68. To WT, Eroh J, Hart J, Vanneste S.. Exploring the effects of anodal and cathodal high definition transcranial direct current stimulation targeting the dorsal anterior cingulate cortex. Sci Rep 2018;8(1):4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rawji V, Ciocca M, Zacharia A, et al. tDCS changes in motor excitability are specific to orientation of current flow. Brain Stimulat 2018;11(2):289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Leinenga G, Langton C, Nisbet R, Götz J.. Ultrasound treatment of neurological diseases–current and emerging applications. Nat Rev Neurol 2016;12(3):161–74. [DOI] [PubMed] [Google Scholar]
- 71. Legon W, Ai L, Bansal P, Mueller JK.. Neuromodulation with single-element transcranial focused ultrasound in human thalamus. Hum Brain Mapp 2018;39(5):1995–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hameroff S, Trakas M, Duffield C, et al. Transcranial ultrasound (TUS) effects on mental states: A pilot study. Brain Stimulat 2013;6(3):409–15. [DOI] [PubMed] [Google Scholar]
- 73. Esmaeilpour Z, Marangolo P, Hampstead BM, et al. Incomplete evidence that increasing current intensity of tDCS boosts outcomes. Brain Stimulat 2018;11(2):310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nikolin S, Huggins C, Martin D, Alonzo A, Loo CK.. Safety of repeated sessions of transcranial direct current stimulation: A systematic review. Brain Stimulat 2018;11(2):278–88. [DOI] [PubMed] [Google Scholar]
- 75. Schmidt SL, Iyengar AK, Foulser AA, Boyle MR, Fröhlich F.. Endogenous cortical oscillations constrain neuromodulation by weak electric fields. Brain Stimulat 2014;7(6):878–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mariano TY, Urman RD, Hutchison CA, Jamison RN, Edwards RR.. Cognitive behavioral therapy (CBT) for subacute low back pain: A systematic review. Curr Pain Headache Rep 2018;22(3):15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.