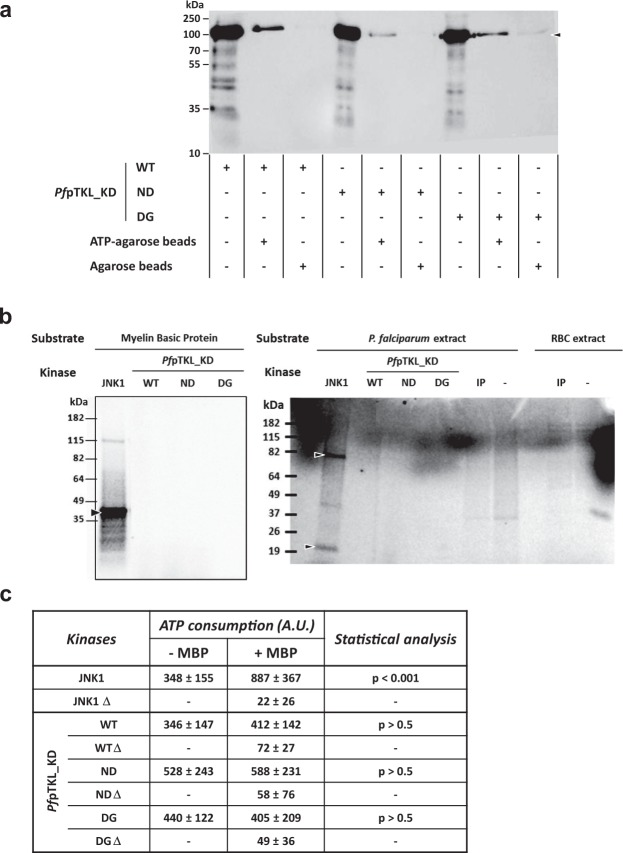
Figure 4.
PfpTKL kinase domain (pTKL_KD_WT) and its mutants (pTKL_KD_ND and pTKL_KD_DG) bind ATP but do not display any kinase activity in vitro. (a) Plasmodium pTKL kinase domain binds ATP. Wild-type and mutated recombinant pTKL kinase domains were incubated with ATP-agarose or agarose beads. Eluates were separated by SDS-PAGE and His6-tagged proteins bound to the beads were detected by western blot. (b) Plasmodium pTKL kinase domain does not catalyze kinase reactions. Kinase reactions performed with γ33P-ATP were stopped by the addition of loading buffer and separated by SDS-PAGE. Gels are displayed according to the substrate: myelin basic protein (left panel), P. falciparum and erythrocyte extracts (right panel). WT = wild-type recombinant kinase domain, ND = N1272D mutant and DG = 1290DFG1292 to 1290GFE1292 mutant. IP = immunoprecipitated PbpTKL-AID-HA. JNK1 was used as an active kinase control. (c) Plasmodium pTKL kinase domain binds ATP but does not display any kinase activity. WT and mutant recombinant pTKL kinase domains were distributed in plates in the presence or absence of myelin basic protein used here as a substrate. JNK1 was used as an active kinase control and all kinases were also heated (∆) to generate negative controls. After incubation at 37 °C for 2 h, the quantity of free ATP remaining in wells was evaluated by luminometry. The ATP consumption displayed in the table corresponds to the difference between the luminometry value obtained without kinase and the luminometry value obtained with the maximum amount of kinase (80 ng/well for JNK1, and 8 µg/well for PfpTKL) (mean ± SD; three biological replicates and three technical replicates per data point – raw data are available in Supplementary Table S4).