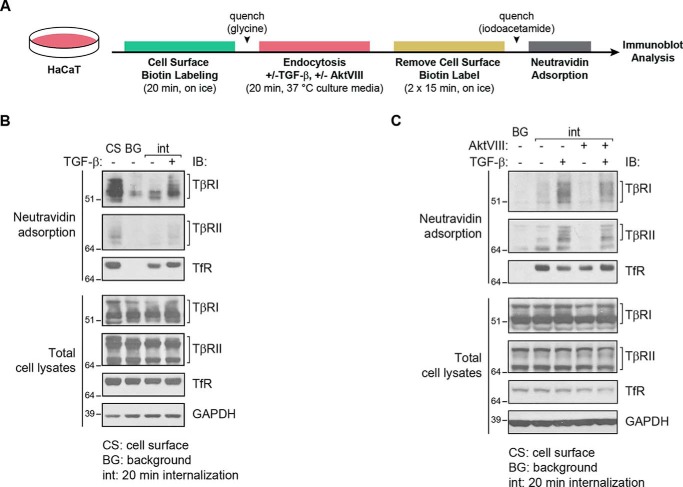
Figure 4.
TGF-β increases internalization of TβRI and TβRII in an Akt-dependent manner. TGF-β receptor internalization (int) analysis of HaCaT cells in the presence of TGF-β1 and/or AktVIII or vehicle control. A, schematic diagram of the experimental flow used to generate the results in B and C. The cells were subjected to cell-surface protein biotinylation for 20 min on ice and then switched to cell culture medium under normal growth conditions at 37 °C for 20 min, in the presence or absence of TGF-β1 and/or AktVIII. The biotin label was then removed from proteins at the cell surface using GSH, and the internalized biotinylated proteins, which were protected against removal of the biotinylation, were purified by affinity adsorption to NeutrAvidin. B, biotin-labeled proteins, purified by NeutrAvidin adsorption, were visualized by SDS-PAGE and immunoblot (IB) analysis. Lane 1 shows total cell-surface labeled protein prior to incubation at 37 °C and reversal of biotinylation (cell surface, CS). Lane 2 shows background (BG) levels of biotinylated proteins in cells kept on ice (0 min at 37 °C) prior to reversal of biotinylation, as control for those samples that were subsequently switched to 37 °C. The third and fourth lanes show internalized TβRI and TβRII receptors after 20 min at 37 °C, in the absence or presence of TGF-β, after reversal of biotinylation. TβRI and TβRII internalization was enhanced in response to TGF-β1. C, using the same treatment regimen, the presence of AktVIII during incubation at 37 °C attenuated the TGF-β-stimulated internalization of TβRI and TβRII. TfR and GAPDH levels in cell lysates serve as loading controls.