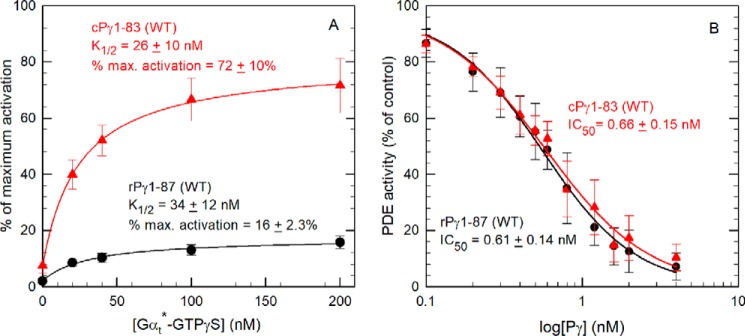
Figure 3.
PDE6 reconstituted with cPγ is more effectively activated by Gtα*-GTPγS than PDE6 containing rPγ. A, Gtα*-GTPγS activation assay. 1 nm Pαβ was preincubated with a 10-fold molar excess of WT rPγ or cPγ to reconstitute the PDE6 holoenzyme. Gtα*-GTPγS was then added at the indicated concentrations and incubated for 1 h prior to measuring PDE activity with 2 mm cGMP as substrate. PDE activity is reported relative to the activity of Pαβ in the absence of Pγ. The data are the mean (± S.D.) for 19 or 20 separate determinations of PDE6 reconstituted with WT rPγ or cPγ, respectively. The data were fit by nonlinear regression analysis using a three-parameter hyperbolic equation and are reported in Table 1; basal activities for PDE6 reconstituted with rPγ and cPγ were 2% ± 0.8% and 8% ± 3%, respectively. B, Pγ inhibition assay. Pαβ (0.2 nm) was incubated with the indicated concentrations of WT rPγ or cPγ for 10 min, and then the catalytic activity was measured using 2 mm cGMP as substrate. The data are the mean (± S.D.) of eight experiments with rPγ and cPγ. A three-parameter logistic equation was used to estimate the IC50: rPγ, IC50 = 0.56 nm; cPγ, IC50 = 0.58 nm.