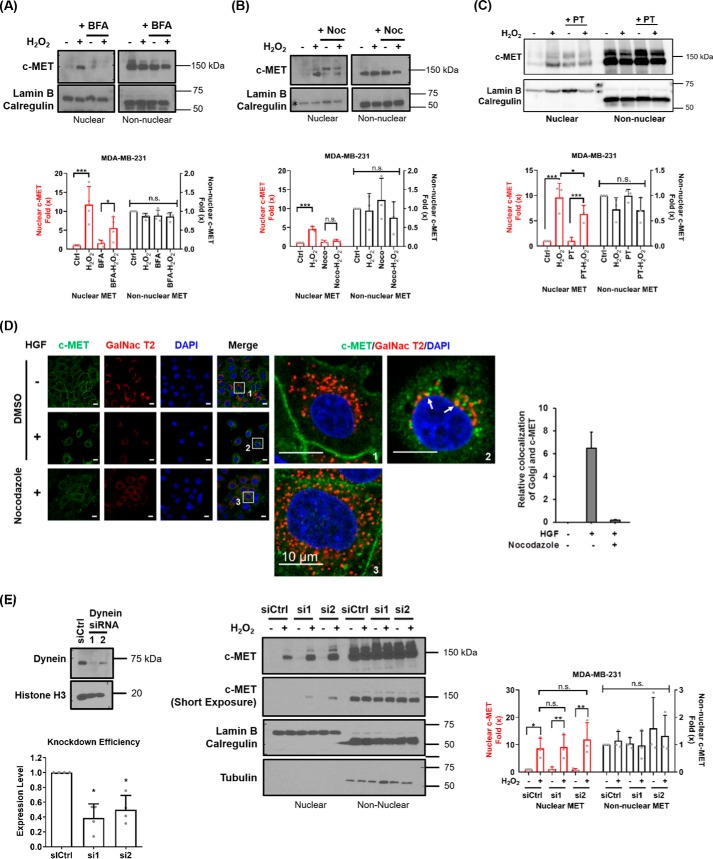
Figure 2.
Nuclear accumulation of full-length c-MET can be inhibited by Golgi and microtubule disruption. A, MDA-MB-231 cells were treated with 5 μm BFA for 30 min prior to 30-min 10 mm H2O2 treatment before fractionation. Lamin B and calregulin were used as markers for nuclear and non-nuclear fractions, respectively. Fold change of MET from four independent experiments are summarized in histograms as means ± S.D. B, MDA-MB-231 cells were treated with nocodazole (Noc) for 30 min before H2O2 stimulation and cellular fractionation. Normalized fold change of MET from three independent experiments are shown as means ± S.D. in histograms. C, MDA-MB-231 cells were treated with 1 μm paclitaxel (PT) for 4 h before H2O2 stimulation and cellular fractionation. Normalized fold change of MET from three independent experiments are shown as means ± S.D. in histograms. D, HeLa cells were treated with solvent (DMSO), with HGF (+) or without HGF (−), and nocodazole before fixation for immunofluorescence staining for c-MET (green), GalNac T2 (red), and DAPI (blue). Insets show enlarged views of nuclear c-MET localization. Scale bars, 10 μm. E, left panel, knockdown of dynein by transient transfection of two different siRNAs (Dynein siRNA 1 and 2) in MDA-MB-231 cells. Right panel, control and dynein knockdown MDA-MB-231 cells were treated with H2O2 and subjected to fractionation followed by Western blotting analysis. Lamin B and calregulin were used as markers for nuclear and non-nuclear fractions, respectively. Knockdown efficiencies from four experiments are shown in histograms as means ± S.D. Fold changes (×) of three independent experiments are indicated in histograms as means ± S.D.