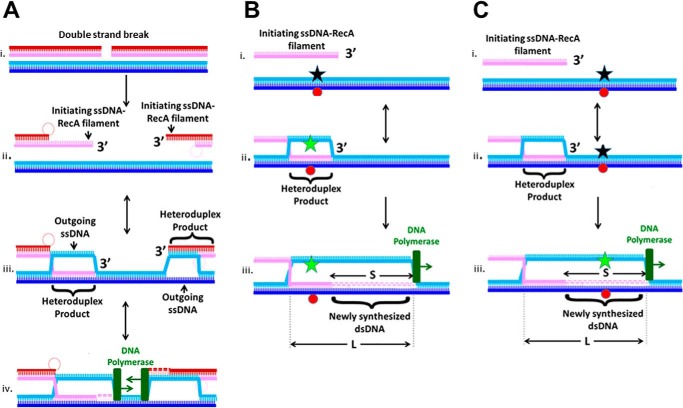
Figure 1.
Schematics of in vivo double-strand break repair and in vitro assays proving heteroduplex formation or extension of the invading strand in a D-loop. A, schematic of in vivo repair. Panel i, initial double-strand break occurs in the DNA with the red and pink backbones. Panel ii, formation of ssDNA–RecA filaments on the ssDNA at the 3′ ends of the broken dsDNA. The illustration also shows degradation of the invading strands flanking the break, which occurs if the RecBCD pathway is followed. Panel iii, interactions between the ssDNA–RecA filaments and the unbroken dsDNA (blue strands) create heteroduplex dsDNA that pairs the invading and complementary strands, leaving the outgoing strand unpaired. Panel iv, after the heteroduplex reaches the 3′ end of the invading strand, the DNA polymerase can use the complementary strand as a template to extend the 3′ end of the invading strand. B, panels i–iii, in vitro monitoring of the heteroduplex product using FRET due to a fluorescein molecule (star) on the outgoing strand and a rhodamine molecule on the complementary strand (red circle). Black stars represent quenched fluorescein. Green stars indicate fluorescein molecules with no FRET. Formation of a heteroduplex product in the sequence region containing the fluorophores separates the outgoing and complementary strands, which increases fluorescein emission. The DNA polymerase is shown in green. It can perform strand displacement synthesis that extends the invading strand in the D-loop. C, panels i–iii, in vitro monitoring of the dsDNA structure beyond the 3′ end of the invading strand using FRET. Heteroduplex formation does not significantly increase emission, but strand displacement and DNA synthesis performed by the DNA polymerase can enhance emission.