Abstract
Collective motion by animal groups can emerge from simple rules that govern each individual's interactions with its neighbours. Studies of extant species have shown how such rules yield coordinated group behaviour, but little is known of their evolutionary origins or whether extinct group-living organisms used similar rules. Here, we report evidence consistent with coordinated collective motion in a fossilized group of the extinct fish Erismatopterus levatus, and we infer possible behavioural rules that underlie it. We found traces of two rules for social interaction similar to those used by extant fishes: repulsion from close individuals and attraction towards neighbours at a distance. Moreover, the fossilized fish showed group-level structures in the form of oblong shape and high polarization, both of which we successfully reproduced in simulations incorporating the inferred behavioural rules. Although it remains unclear how the fish shoal's structure was preserved in the fossil, these findings suggest that fishes have been forming shoals by combining sets of simple behavioural rules since at least the Eocene. Our study highlights the possibility of exploring the social communication of extinct animals, which has been thought to leave no fossil record.
Keywords: swarm behaviour, shoaling, self-propelled particles, self-organization, trace fossil, ichnology
1. Introduction
Group-living animals often show coordinated collective motion which can be explained by local interactions among group members [1]. Empirical and theoretical studies have found support for various interaction rules, with repulsion from close neighbours and alignment and/or attraction towards distant neighbours often playing essential roles in coordination [2–7]. These interaction rules can explain patterns of collective behaviour in diverse extant taxa, including bird flocks, fish schools and insect swarms [2,8,9]. Fossils indicating mass mortality suggest that many extinct species also lived in groups [10], but nothing is known of their collective motion or the interaction rules underlying it. This limits our understanding of the evolutionary steps towards complex and coordinated collective behaviour.
Behaviour is a dynamic process that leaves a scarce fossil record, which hinders our understanding of the interactions of past organisms with one another and their environment. Some insight can be gained from trace fossils produced by biological activity, such as footprints, burrows and bite marks [11,12]. Although these records lack the temporal component of behaviour, they provide snapshots of spatial patterns from which behavioural rules of extinct animals can be inferred. For example, analysis of fossilized trails of benthic marine organisms identified self-avoiding behavioural rules that lead to Lévy-like movement patterns [13]. For collective motion of animals, a fossil of mass mortality can preserve individual positions and heading directions, providing a snapshot of group dynamics and a ‘trace’ of how individuals were interacting with each other.
A window on ancient shoaling behaviour may be found in fossilized aggregations of fish whose positions and attitudes are preserved as if the groups were fixed in a moment [10]. These fossils have been interpreted as shoaling groups because of the similar size of preserved individuals, a basic characteristic of current fish shoals. However, no effort has been made to analyse or model these aggregations from the perspective of collective behaviour, with the goal of understanding the interactions leading to shoaling behaviour by extinct animals. In this study, we inferred possible behavioural rules used by a group of fossilized extinct Erismatopterus levatus by analysing their positions and heading directions. Using estimated behavioural rules, we developed a simulation model of collective motion and then compared group-level patterns of the simulated shoal with those of the fossilized fish group.
2. Material and methods
(a). Geological setting and fossil identification
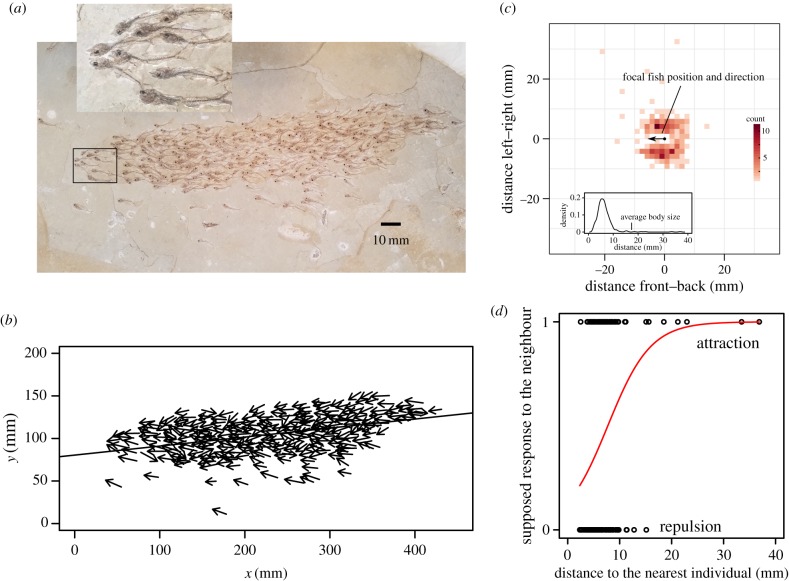
According to the label of specimen FPDM-V8206 (Fukui Prefectural Dinosaur Museum), the slab was collected in the USA and formed during the Eocene. We further estimated that the specimen was from the Green River Formation based on the colour of the matrix, which is greyish limestone shale. Moreover, E. levatus is known only from Lake Gosiute and Lake Uinta deposits of the Green River Formation [14–17] (electronic supplementary material, figures S1 and S2). The size of the specimen was 570 × 375 mm, and it contained 259 percopsid fish (figure 1a). We identified the species as E. levatus based on their dorsal fin rays having two spines with six to seven soft fin rays, the base of their pelvic fin having a subthoracic location, and their anal fin rays having two spines with seven soft fin rays.
Figure 1.
Collective behaviour in a fossil fish school. (a) Analysed fossil of a group of E. levatus in specimen FPDM-V8206 from the Eocene (Green River Formation). (b) Representation of the length and heading of each fish. The long line passes through the centre of the group (average x and y) in the direction of the average heading. (c) Frequency distribution of each fish's nearest neighbour, showing that most are positioned laterally. Inset shows the density plot of the distance between individuals. (d) The projected short-term change of the distance to the nearest individual as a function of the observed starting distance. Initially, close neighbours tend to move away (repulsion) while initially distant neighbours move closer (attraction). Red line indicates fitted logistic regression. (Online version in colour.)
(b). Data analysis
The slab contained fossils of 259 fish, of which two individuals were dropped from the analysis because they were apart from the central aggregation. Specifically, they were twice the average body length from their nearest neighbours, and there were no fish present in the direction that they were heading or at their sides (electronic supplementary material, figure S3). We measured heading direction of each of 257 fish as a vector from the base of the tail to the tip of the upper jaw (electronic supplementary material, figure S4). As the backbones of many individuals were not straight, the length of the heading direction vector was not necessarily equal to the body length (electronic supplementary material, figure S4). Thus, we measured body length by approximating it with two lines of the same length, where we defined the intersection of these two lines as the position of each individual (electronic supplementary material, figure S4). Actual fish shoals move in three-dimensional space, while the fossilized fish were distributed on the two-dimensional surface of the slab. We assumed that this is a two-dimensional projection of a fish shoal that was compressed onto a horizontal plane, and we analysed the positions and the directions of fish two-dimensionally. As a result of this approximation, we may underestimate the distance between neighbouring individuals. However, we expect that the relationship between neighbours should be qualitatively robust, given that vertical distances are often smaller than frontal or horizontal distances [18]. If the living shoal of E. levatus was swimming in a shallow lake, the appearance of the fossilized fish group should correspond to the shoal observed from above, as in previous empirical studies [3]. To examine the interactions between neighbouring individuals, we first measured the distance from the position of each individual to its nearest neighbour. Then, we moved each individual towards its heading direction by a very small distance proportional to its body length (0.0001 mm × body length/average body length). All fish were moved at the same time, and we inferred their behaviour at the next moment by measuring changes of the distance between nearest neighbours: becoming smaller (attraction) or larger (repulsion). To test if the distance to the nearest neighbour affected their behaviour, we used a generalized linear model (GLM) with binomial errors and logit link (logistic regression), where their behaviour (attraction: 1, repulsion: 0) was the dependent variable and the distance to the nearest neighbour was the explanatory variable. A likelihood ratio test was used to test for a significant effect (type II test). We also performed this analysis by fitting a GLM to surrogate datasets (n = 1000 randomizations), for which we randomized the heading directions among individuals. We obtained coefficients (slopes) for all datasets and compared them with that of the fossilized group.
To describe the collective structure of the fish shoals, we measured the elongation and polarization of the group. To measure the elongation, we rotated the group by the average angle of heading directions, to create a bounding box around the school aligned to the average heading direction. We measured the length–width ratio of the bounding box as the index of elongation. We also measured the polarization parameter ρ, which is a measurement of how aligned the individuals in a group are. All analyses were performed using R v. 3.1.3 (https://cran.r-project.org/).
(c). Fish distribution with direction preference
To examine if alignment of individuals caused by water currents could explain the spatial distribution of the fossilized fish group, we created artificial datasets. In each dataset, the observed individual positions were maintained, while each heading direction was first set to the observed average heading and then rotated by an angle drawn from a Gaussian distribution with a mean of zero. We varied the standard deviation of this distribution from 0.01 (high directional preference) to 1.0 (low directional preference). For each value of s.d., we created 100 artificial datasets. We then subjected each dataset to the same logistic regression that had revealed for the fossilized group a pattern of repulsion at short distances and attraction at long distances. Each dataset yielded a coefficient linking distance to movement, which we then compared to the coefficient of the fossilized fish group.
(d). Simulations
To test if the interaction rules observed in the fossilized fish group can explain its collective structure, we developed an individual-based model based on published zone models of fish shoal movements [2,19]. These are called zone models because individuals respond to neighbours within a set of interaction zones. When appropriate interaction rules are specified, zone models are known to generate the highly polarized or oblong patterns that we observed in the fossilized fish shoal [2,20]. In our simulations, 257 fish moved in a two-dimensional space with periodic boundary conditions (size = L2). We chose periodic boundary conditions to avoid separation of the group. We used a large enough space to ensure that individuals at the front and back of the group did not interact with each other. Simulated fish followed two behavioural rules: (i) repulsion to maintain a minimum distance between themselves and other individuals within a zone of radius rz; and (ii) attraction-and-alignment towards neighbours within an interaction zone of radius ra, if the fish is not performing an avoidance behaviour. In our analysis of the fossilized shoal, we found evidence for both attraction and repulsion behaviour (see Results), but we could not find a way to test for traces of alignment behaviour from a single snapshot of collective movement. However, most extant fish species align themselves via direct alignment behaviours [5,6] or by combining attraction and repulsion behaviours [3,4], suggesting that it is reasonable to assume that E. levatus also has some alignment mechanism. In zone models, the most effective way to account for alignment is by specifying an alignment behaviour for a particular zone [2]. Thus, we included an alignment term additive to the attraction rule, as in a previous study [19]. Based on these rules, movement direction of each fish was obtained as follows. If there were other fish j within a distance rz of fish i, then fish i avoided these fish by turning towards direction
while if there were no fish within its repulsion zone, the fish i responded to fish k within the interaction range ra by turning towards direction
where ci(t) and vi(t) represent the coordinates and velocity, respectively, of fish i at time t. If there were no fish in either the repulsion or attraction-and-alignment zones, the fish continued to move in the same direction as the previous time step. Fish could turn at a maximum rate of θΔt degrees. Thus, if the angle between vi(t) and di(t + Δt) was less than θΔt, fish i could proceed in its desired vector, otherwise it turned θΔt towards it. All turning was assumed to be subject to slight error, which was simulated by rotating vi(t + Δt) by a random angle drawn from a normal distribution with mean zero and standard deviation σ. Once its direction was determined, the fish moved with a speed of , which was limited to the range given in the electronic supplementary material, table S1. That is, if fell outside this range, the speed was set to the closest extreme of the range. The next coordinate of fish i was . We performed 1000 simulation runs and collected a snapshot of the data at time step 10 000. For each snapshot, we performed the same analysis as with the fossilized group. The values or ranges used in our simulations are summarized in the electronic supplementary material, table S1. We chose these parameter values arbitrarily from ranges used in a previous study [2]. This is because the purpose of our simulation was to test if the inferred potential behavioural rules are capable of recreating patterns similar to those observed in the fossilized fish shoal. As the information we can obtain from the fossil is limited, we did not try to estimate parameter values from the fossilized shoal. For example, it is nearly impossible to measure the moving speeds or perceptional ranges of individuals. The simulation program was implemented in Microsoft Visual Studio C++ 2017.
3. Results and discussion
The analysed slab contained 257 individuals of E. levatus, most of them heading in similar directions (figure 1a,b). All individuals were in the same lamination. Body lengths (standard length) ranged from 10.57 to 23.54 mm, which is much smaller than the descriptive specimen of this species (approx. 65 mm) [21], indicating that they were juveniles or larvae. The distribution of the distance to the nearest neighbour showed a single peak with a value smaller than the average body length (figure 1c). Moreover, most of the nearest neighbours were positioned laterally (figure 1c). This non-uniform structure (anisotropy) itself may be an effect of local interactions between nearest neighbours, probably caused by the lateral visual field and elongated body shape along the direction of motion [7]. Similar features are also observed in the nearest neighbour analysis of extant shoaling fish species, although details vary among species [3,6,22–26]. Note that the observed distances between nearest individuals might be smaller than those seen in extant fishes [18], because of the compression of three-dimensional structures into two dimensions during fossilization.
To identify interaction rules of individuals in the group, we examined how distance to the nearest individual would change at the next moment after the snapshot preserved on the slab. We inferred the position of each fish if it moved a very short distance in its heading direction from its preserved position. Each fish was then classified by whether it was now closer to its nearest neighbour (attraction) or further from it (repulsion). When we examined how the starting distance to the nearest neighbour affected their response, we found repulsion at short distances, switching to attraction as distance increased (logistic regression: slope ± s.e. = 0.243 ± 0.062, likelihood ratio test: χ21 = 23.249, p < 0.001; figure 1d). This trend completely disappeared if we analysed surrogate datasets created by randomly switching heading directions among individuals (slope: mean ± s.d. = 0.002 ± 0.037, n = 1000), indicating that the observed association between nearest neighbour distance and heading direction did not happen by chance. This analysis reveals the fossilized trace of at least two interaction rules: repulsion at short distances to avoid collisions by ensuring a minimum space around each fish, and attraction at longer distances to maintain group cohesion. These two behavioural rules can play an important role in coordinated collective motion of some extant fish species [3,4].
The structure of the fossilized fish group also shared characteristics with extant fish shoals. The group showed an oblong shape longer in the direction of movement (length–width ratio of the bounding box was 3.171). This is the usual shape of extant shoals [27,28] and is thought to protect against ambush predators by reducing the frontal area, where these predators tend to attack [27]. The fossilized group also showed a high level of individual alignment to one another, as measured by the polarization parameter ρ. This parameter is calculated by dividing the length of the sum of individual unit vectors in the direction of motion of each individual, ui, by the number of group members. That is where N is the number of group members. Polarization ranges from 0 (no alignment on average) to 1 (all headings are parallel) [2]. The fossilized group was highly polarized (ρ = 0.902), which corresponds to one of the stable states of collective motion observed in extant fish shoals [2,29].
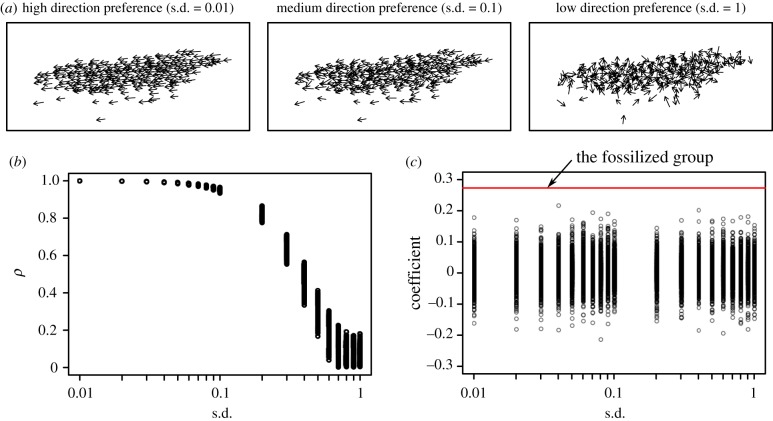
An alternative hypothesis is that apparent shoaling patterns are an incidental result of a taphonomic process. A purely random process cannot provide an adequate explanation, as the traces of interaction rules described above disappeared if fish positions were randomized (electronic supplementary material, figure S5). The fish shoal fossil contrasts with other slabs of fish mass mortality that show scattered distributions with no evidence of interaction rules (electronic supplementary material, figure S6). In previous studies, well-aligned assemblies of fish like the one we studied have been thought to reflect sorting of fish carcasses by water currents and wind [30–32]. However, this cannot adequately explain our fossil for the following reasons. First, although wind or water currents are expected to sort the fish into size classes [32], we found no correlations between fish position and body length (linear model; heading direction: F = 2.527, p = 0.113; crossing heading direction: F = 1.618, p = 0.205). (It should be noted, this lack of correlation is also unexpected for fish shoals, where size sorting is common [33].) Second, directional preference alone cannot explain the trace of interaction rules (figure 2). When we allocated new headings to all fish on the slab by randomly selecting them from a normal distribution; we found that higher directional preference (lower standard deviation of heading) led to higher polarization (figure 2b) but did not reproduce the observed pattern of repulsion at shorter distances and attraction at longer distances (figure 2c). Third, water currents often result in the disintegration of small fin bones from the fish carcasses, which are expected to be sorted into size classes [34,35], but there were no scattered paired bones and elements in our specimen. All in all, these results discount explanations based on sorting of fish carcasses, suggesting that the observed pattern may instead reflect fish behaviour.
Figure 2.
Collective structures of simulated fish groups that vary in directional preference. (a) Visualized examples varying in preference. (b) Polarization metrics of simulated groups. With high directional preference (lower standard deviation), groups showed high polarization (greater than 0.9), like the fossilized fish group and simulated fish shoals (figures 1b and 3c). (c) Coefficients for logistic regression of inferred behaviour (attraction or repulsion) on nearest neighbour distance, for simulated groups. Coefficients were evenly distributed around 0, indicating no relationship between behaviour and distance. This contrasts with the fossilized fish group (figure 1d), whose positive coefficient (shown by the red line) indicates a shift from repulsion to attraction as distance increases. For each value of s.d., we created 1000 datasets. (Online version in colour.)
Fossils with animals preserved while doing something are referred to as ‘frozen behaviours’ [10]; examples include fighting dinosaurs [36], queueing trilobites [37] and insects in copulation [38]. These fossils are assumed to result from rapid burial, which preserves individual positions during interactions. In the present study, we similarly assume that our fish shoal was fixed near instantaneously so that individual positions and heading directions were preserved in the fossil. This taphonomic process must have been rapid enough to maintain the trend of the interaction rules, but it appears not to have preserved the structures of the shoal completely. The presence of several abnormal individuals heading in opposite directions suggests that their positions were somehow modified (electronic supplementary material, figure S3). Rapid fixation of the fish shoal might be possible by sand dune collapse on shallow water, which can produce a bed in only seconds or minutes [39]. Unfortunately, we could not obtain any evidence to support the occurrence of this event from the specimen, which consists of a very thin slab giving no information about the overlying or underlying layers or the entire bedding structure. To estimate how the fish shoal was fixed would require comprehensive geological information about the rocks surrounding the fossil, including the sequence of sedimentary structures, stratification patterns and lithic characteristics, which would be possible by fieldwork (e.g. as in [37]). Sudden freezing caused by supercooling could also explain rapid fixation, although this seems unlikely, given the warm climate estimated for the Eocene Green River Formation [40].
Despite lacking evidence of catastrophic events, we can use geological information from the fossiliferous rock to infer the fossilized environment. For example, the slab comprises thin parallel laminae, without any ripples or bedforms. This suggests that the body of water had a flat, even bottom without a pronounced depression. If so, then surface heterogeneity cannot explain the dense aggregation of fish in the specimen. Considering that dead bodies in an assemblage of carcasses would be positioned all over the slab (this is true for other mass mortality fossils [10,31,32] and electronic supplementary material, figure S6), the observed localized aggregation is likely to be the result of behaviour rather than an artefact of fossilization (see [41] for a discussion of this issue). Also, the sediment is fine-grained mud, which is one criterion for an in situ rather than transported assemblage [42,43]. Nevertheless, we cannot completely exclude the possibility that the aggregation of fish was caused by the accumulation of dead bodies over a short time rather than a simultaneous fixation of an entire shoal. Further analysis of similar specimens of mass mortality may be useful to distinguish these explanations.
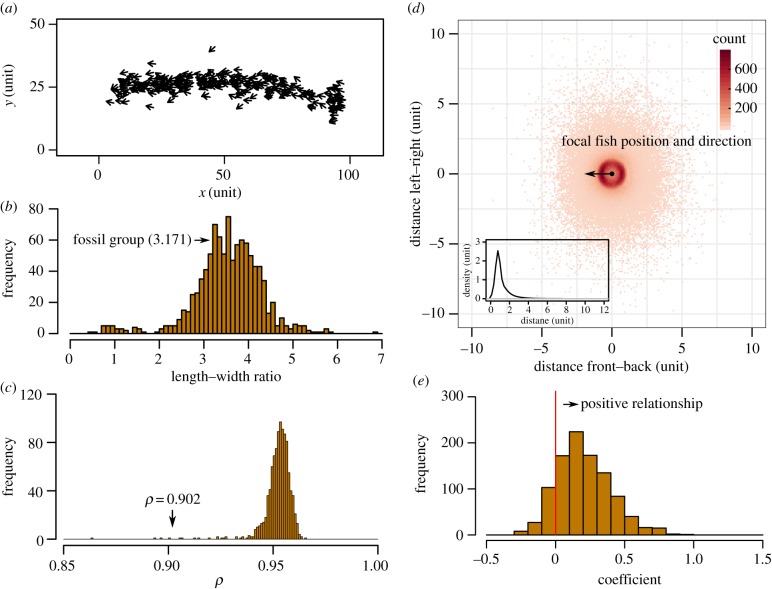
To test if our inferred interaction rules can explain the spatial patterns preserved in the fossilized fish group, we built a simple simulation with self-propelled particles based on models of extant fish shoals [2,19]. In our simulations, 257 fish moved in two-dimensional space, following two behavioural rules: (i) repulsion to maintain a minimum distance between individuals; and (ii) attraction-and-alignment towards neighbours if individuals are not performing an avoidance behaviour (see Material and methods). We performed 1000 simulation runs and collected data on a snapshot of each run. We then examined the snapshots for collective structures similar to the fossilized fish group, and we tested whether we could infer the same behavioural rules from the snapshots.
Most of our simulated fish shoals showed oblong shapes (figure 3a,b) and high polarization (figure 3a,c), and the observed values of the fossilized group fell well within the range of simulated values. Moreover, from the snapshots of our simulations, we could find the trace of two interaction rules just as we did for the fossilized fish group. We found that the distribution of the distance to the nearest individual showed a single steep peak (figure 3d). By assuming that each fish moved a very short distance in its current heading, most snapshots showed a positive relationship between the distance to the nearest individual and the probability of approaching it (862 of 1000 snapshots showed a positive relationship, where 33.93% of them were statistically significant; figure 3e). However, one difference between the fossilized fish shoal and our simulation is the distribution of relative positions of nearest neighbours. There are many more nearest neighbours at the front and back of an individual in our simulations (figure 3d) than in the fossilized fish shoal (figure 1c). This is probably because actual fish have body shapes that are elongated along the direction of motion, while the agents in our simulations are simply points without physical bodies [7].
Figure 3.
Simulated fish shoal. (a) A representative snapshot of simulated fish. (b) The frequency distribution of the length–width ratio of the bounding box of simulated fish shoals (n = 1000). Groups showed an oblong shape longer in the direction of movement (length–width ratio > 1). The value of the fossilized fish group fell near the distribution centre. (c) The frequency distribution of polarization values of simulated shoals (n = 1000). The value of the fossilized fish group was 0.902. (d) The frequency distribution of each simulated fish's nearest neighbour. Inset shows the density plot of the distance between individuals (1000 replicates are pooled). (e) Inferring interaction rules from snapshots of simulated fish shoals. In 862 of 1000 snapshots, there was a trend for individuals to switch from repulsion to attraction as distance to nearest neighbour increased (i.e. a positive logistic regression coefficient). (Online version in colour.)
One of the main functions of shoaling behaviour is reducing predation risk through risk dilution and predator confusion [44]. To achieve effective avoidance of predation, it has been demonstrated that prey should move towards and align with their neighbours, to prevent being solitary or at the edge of the group [45]. Because we found evidence of approach from a distance in our fossilized group of E. levatus (figure 1d), we can reasonably infer predator avoidance as a selective pressure leading to shoaling behaviour. Consistent with this, the density within the group was higher in the safer central area, while it was lower at the edge of the group (electronic supplementary material, figure S7), where predators often attack [45]. Thus, juveniles and larvae of E. levatus might have experienced a high predation pressure in their Eocene intermountain lake habitat. Indeed, various predatory fishes, including catfish, gar and predatory Diplomystus, have been reported from the same geological formation (Lakes Gosiute and Uinta of the Green River Formation [15,17]). Moreover, half of extant fish species shoal as juveniles, when they are more vulnerable to predation [46]. Thus, analysing mass mortality fossils from the viewpoint of collective behaviour can provide insight into selection pressures acting in ancient food webs.
An important goal of collective behaviour research is to identify the evolutionary process leading to coordinated collective motion because different interaction rules can be selected under different ecological conditions and historical contingencies [47]. For this purpose, the comparative analysis of the interaction rules adopted by different species can be an effective approach. In this study, we found repulsion and attraction rules in a fish species belonging to the order Percopsiformes, which is phylogenetically distinct from orders of extant species showing similar interaction rules, such as Cypriniformes (Notemigonus crysoleucas [3] and Danio rerio [48]), Cyprinodontiformes (Gambusia holbrooki [4] and Poecilia reticulate [5]) or Characiformes (Pristella maxillaris [6]), according to a recent phylogeny [49]. This suggests that E. levatus living in an Eocene freshwater system experienced a similar evolutionary process and occupied a similar niche to that of the extant shoaling Cypriniformes, Cyprinodontiformes or Characiformes species. Inferring behaviour from fossils of these fish is a valuable source of information about the evolution of shoaling, considering that almost all species in Percopsiformes, including E. levatus, are extinct. By applying our approach not only to other fossilized fish groups but also to other taxa whose fossils of mass mortality can be found (e.g. shrimp, mammals and dinosaurs [10]), we can better understand palaeoecology and achieve the reconstruction of the evolutionary process behind coordinated collective motion.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Soki Hattori and Soichiro Kawabe for help in accessing the specimen; Masato S. Abe, Yoichi Azuma, Kaitlin M. Baudier, Shigeto Dobata, Takuya Imai, Kenji Matsuura, Hiraku Nishimori, Thodore P. Pavlic and Masashi Shiraishi for fruitful discussion; Satoshi Maruyama and Keiichi Takeuchi for providing the opportunity for collaboration between N.M. and S.M.; and two anonymous reviewers for comments on an earlier version of the manuscript.
Data accessibility
The data supporting this article and the codes for simulations can be found in the electronic supplementary material.
Authors' contributions
N.M. and S.M. conceived and designed the study. N.M. and S.M. collected the data. S.M. collected geological information. N.M. performed data analysis and computer simulations. N.M., S.M. and S.C.P. interpreted the results. N.M. drafted the manuscript and S.M. and S.C.P. edited the manuscript. All authors gave final approval for publication.
Competing interests
The authors declare no competing interests.
Funding
N.M. is supported by a JSPS Overseas Research Fellowship.
References
- 1.Camazine S, Deneubourg J-L, Franks NR, Sneyd J, Theraulaz G, Bonabeau E. 2001. Self-organization in biological systems. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Couzin ID, Krause J, James R, Ruxton GD, Franks NR. 2002. Collective memory and spatial sorting in animal groups. J. Theor. Biol. 218, 1–11. ( 10.1006/jtbi.2002.3065) [DOI] [PubMed] [Google Scholar]
- 3.Katz Y, Tunstrom K, Ioannou CC, Huepe C, Couzin ID. 2011. Inferring the structure and dynamics of interactions in schooling fish. Proc. Natl Acad. Sci. USA 108, 18 720–18 725. ( 10.1073/pnas.1107583108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbert-Read JE, Perna A, Mann RP, Schaerf TM, Sumpter DJ. T, Ward AJ. W. 2011. Inferring the rules of interaction of shoaling fish. Proc. Natl Acad. Sci. USA 108, 18 726–18 731. ( 10.1073/pnas.1109355108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbert-Read JE, et al. 2017. How predation shapes the social interaction rules of shoaling fish. Proc. R. Soc. B 284, 20171126 ( 10.1098/rspb.2017.1126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaerf TM, Dillingham PW, Ward AJW. 2017. The effects of external cues on individual and collective behavior of shoaling fish. Sci. Adv. 3, e1603201 ( 10.1126/sciadv.1603201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballerini M, et al. 2008. Interaction ruling animal collective behavior depends on topological rather than metric distance: evidence from a field study. Proc. Natl Acad. Sci. USA 105, 1232–1237. ( 10.1073/pnas.0711437105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buhl J, Sumpter DJT, Couzin ID, Hale JJ, Despland E, Miller ER, Simpson SJ. 2006. From disorder to order in marching locusts. Science 312, 1402–1406. ( 10.1126/science.1125142) [DOI] [PubMed] [Google Scholar]
- 9.Lopez U, Gautrais J, Couzin ID, Theraulaz G. 2012. From behavioural analyses to models of collective motion in fish schools. Interface Focus 2, 693–707. ( 10.1098/rsfs.2012.0033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boucot A. 1996. Evolutionary paleobiology of behaviour and coevolution. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 11.Seilacher A. 1967. Fossil behavior. Sci. Am. 217, 72–80. ( 10.1038/scientificamerican0867-72) [DOI] [Google Scholar]
- 12.Plotnick RE. 2012. Behavioral biology of trace fossils. Paleobiology 38, 459–473. ( 10.1666/11008.1) [DOI] [Google Scholar]
- 13.Sims DW, Reynolds AM, Humphries NE, Southall EJ, Wearmouth VJ, Metcalfe B, Twitchett RJ. 2014. Hierarchical random walks in trace fossils and the origin of optimal search behavior. Proc. Natl Acad. Sci. USA 111, 11 073–11 078. ( 10.1073/pnas.1405966111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchheim HP, Surdam RC. 1977. Fossil catfish and the depositional environment of the Green River Formation, Wyoming. Geology 5, 196–198. () [DOI] [Google Scholar]
- 15.Grande L. 1984. Paleontology of the Green River Formation, with a review of the fish fauna. Bull. Geol. Surv. Wyoming 63, 1–333. ( 10.1021/MA0493168) [DOI] [Google Scholar]
- 16.Grande L. 1994. Studies of paleoenvironments and historical biogeography in the Fossil Butte and Laney Members of the Green River Formation. Univ. Wyoming, Contrib. Geol. 30, 15–32. ( 10.2113/gsrocky.30.1.15) [DOI] [Google Scholar]
- 17.Grande T, Borden WC, Smith WL. 2013. Limits and relationships of Paracanthopterygii: a molecular framework for evaluating past morphological hypotheses. In Mesozoic fishes 5, global diversity and evolution (eds Arratia G, H-Schultz P, Wilson MVH), pp. 385–418. Munchen, Germany: Verlag. [Google Scholar]
- 18.Pavlov D, Kasumyan AO. 2000. Patterns and mechanisms of schooling behavior in fish: a review. Artic J. Ichthyol. 40, 163–231. [Google Scholar]
- 19.Hensor E, Couzin ID, James R, Krause J. 2005. Modelling density-dependent fish shoal distributions in the laboratory and field. Oikos 110, 344–352. ( 10.1111/j.0030-1299.2005.13513.x) [DOI] [Google Scholar]
- 20.Hemelrijk CK, Hildenbrandt H. 2008. Self-organized shape and frontal density of fish schools. Ethology 114, 245–254. ( 10.1111/j.1439-0310.2007.01459.x) [DOI] [Google Scholar]
- 21.Newbrey MG, Murray AM, Wilson MVH, Brinkman DB, Neuman AG. 2013. A new species of the paracanthopterygian Xenyllion from the Mowry Formation (Cenomanian) of Utah, USA. In Mesozoic fishes 5, global diversity and evolution (eds Arratia G, Schultze H-P, Wilson MVH), pp. 363–384. Munchen, Germany: Verlag. [Google Scholar]
- 22.Huth A, Wissel C. 1994. The simulation of fish schools in comparison with experimental data. Ecol. Modell. 75–76, 135–146. ( 10.1016/0304-3800(94)90013-2) [DOI] [Google Scholar]
- 23.Lukeman R, Li Y-X, Edelstein-Keshet L. 2010. Inferring individual rules from collective behavior. Proc. Natl Acad. Sci. USA 107, 12 576–12 580. ( 10.1073/PNAS.1001763107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward AJW, Schaerf TM, Herbert-Read JE, Morrell L, Sumpter DJT, Webster MM. 2017. Local interactions and global properties of wild, free-ranging stickleback shoals. R. Soc. open sci. 4, 170043 ( 10.1098/rsos.170043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raven C, Shine R, Greenlees M, Schaerf TM, Ward AJW. 2017. The role of biotic and abiotic cues in stimulating aggregation by larval cane toads (Rhinella marina). Ethology 123, 724–735. ( 10.1111/eth.12645) [DOI] [Google Scholar]
- 26.Herbert-Read JE, Kremer L, Bruintjes R, Radford AN, Ioannou CC. 2017. Anthropogenic noise pollution from pile-driving disrupts the structure and dynamics of fish shoals. Proc. R. Soc. B 284, 20171627 ( 10.1098/rspb.2017.1627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bumann D, Krause J, Rubenstein D. 1997. Mortality risk of spatial positions in animal groups: the danger of being in the front. Behaviour 134, 1063–1076. ( 10.1163/156853997X00403) [DOI] [Google Scholar]
- 28.Hemelrijk CK, Hildenbrandt H, Reinders J, Stamhuis EJ. 2010. Emergence of oblong school shape: models and empirical data of fish. Ethology 116, 1099–1112. ( 10.1111/j.1439-0310.2010.01818.x) [DOI] [Google Scholar]
- 29.Tunstrøm K, Katz Y, Ioannou CC, Huepe C, Lutz MJ, Couzin ID. 2013. Collective states, multistability and transitional behavior in schooling fish. PLoS Comput. Biol. 9, e1002915 ( 10.1371/journal.pcbi.1002915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martill DM, Brito PM, Washington-Evans J. 2008. Mass mortality of fishes in the Santana Formation (Lower Cretaceous,?Albian) of northeast Brazil. Cretac. Res. 29, 649–658. ( 10.1016/J.CRETRES.2008.01.012) [DOI] [Google Scholar]
- 31.Diedrich CG. 2009. A coelacanthid-rich site at Hasbergen (NW Germany): taphonomy and palaeoenvironment of a first systematic excavation in the Kupferschiefer (Upper Permian, Lopingian). Palaeobiodivers. Palaeoenviron. 89, 67–94. ( 10.1007/s12549-009-0004-6) [DOI] [Google Scholar]
- 32.Pan Y, Fürsich FT, Zhang J, Wang Y, Zheng X. 2015. Biostratinomic analysis of Lycoptera beds from the Early Cretaceous Yixian Formation, western Liaoning, China. Palaeontology 58, 537–561. ( 10.1111/pala.12160) [DOI] [Google Scholar]
- 33.Krause J, Butlin RK, Peuhkuri N, Pritchard VL. 2000. The social organization of fish shoals: a test of the predictive power of laboratory experiments for the field. Biol. Rev. 75, 477–501. ( 10.1111/j.1469-185X.2000.tb00052.x) [DOI] [PubMed] [Google Scholar]
- 34.Elder RL, Smith GR. 1984. Fish taphonomy and paleoecology. Geobios 17, 287–291. ( 10.1016/S0016-6995(84)80183-7) [DOI] [Google Scholar]
- 35.Elder RL, Smith GR. 1988. Fish taphonomy and environmental inference in paleolimnology. Palaeogeogr. Palaeoclimatol. Palaeoecol. 62, 577–592. ( 10.1016/0031-0182(88)90072-7) [DOI] [Google Scholar]
- 36.Barsbold R. 2016. ‘The Fighting Dinosaurs': the position of their bodies before and after death. Paleontol. J. 50, 1412–1417. ( 10.1134/S0031030116120042) [DOI] [Google Scholar]
- 37.Radwański A, Kin A, Radwańska U. 2009. Queues of blind phacopid trilobites Trimerocephalus: a case of frozen behaviour of Early Famennian age from the Holy Cross Mountains, Central Poland. Acta Geol. Pol. 59, 459–481. [Google Scholar]
- 38.Takahashi Y, Sutou M, Yamamoto S. 2017. The compression mating fossil of sciarid fly (Diptera: Sciaridae) from Shiobara, Tochigi Prefecture, Japan. Paleontol. Res. 21, 288–292. ( 10.2517/2016PR031) [DOI] [Google Scholar]
- 39.Boggs S. 2006. Principles of sedimentology and stratigraphy. Upper Saddle River, NJ: Pearson Prentice Hall. [Google Scholar]
- 40.Ferber CT, Wells NA. 1995. Paleolimnology and taphonomy of some fish deposits in ‘Fossil’ and ‘Uinta’ Lakes of the Eocene Green River Formation, Utah and Wyoming. Palaeogeogr. Palaeoclimatol. Palaeoecol. 117, 185–210. ( 10.1016/0031-0182(94)00127-T) [DOI] [Google Scholar]
- 41.Sánchez-García A, Peñalver E, Delclòs X, Engel MS. 2018. Mating and aggregative behaviors among basal hexapods in the early cretaceous. PLoS One 13, e01916669 ( 10.1371/journal.pone.0191669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brandt DS. 1989. Taphonomic grades as a classification for fossiliferous assemblages and implications for paleoecology. Palaios 4, 303 ( 10.2307/3514554) [DOI] [Google Scholar]
- 43.Chambers L, Brandt D. 2018. Explaining gregarious behaviour in Banffia constricta from the Middle Cambrian Burgess Shale, British Columbia. Lethaia 51, 120–125. ( 10.1111/let.12231) [DOI] [Google Scholar]
- 44.Pitcher TJ. 1986. Functions of shoaling behaviour in teleosts. In Behaviour of teleost fishes (ed. Pitcher TJ.), pp. 294–337. Boston, MA: Springer. [Google Scholar]
- 45.Ioannou CC, Guttal V, Couzin ID. 2011. Uninformed individuals promote democratic consensus in animal groups. Science 334, 1578–1580. ( 10.1126/science.1210280) [DOI] [PubMed] [Google Scholar]
- 46.Shaw E. 1978. Schooling fishes: the school, a truly egalitarian form of organization in which all members of the group are alike in influence, offers substantial benefits to its participants. Am. Sci. 66, 166–175. [Google Scholar]
- 47.Wood AJ, Ackland GJ. 2007. Evolving the selfish herd: emergence of distinct aggregating strategies in an individual-based model. Proc. R. Soc. B 274, 1637–1642. ( 10.1098/rspb.2007.0306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hinz R, Polavieja GG. 2017. Ontogeny of collective behavior reveals a simple attraction rule. Proc. Natl Acad. Sci. USA 114, 2295–2300. ( 10.1073/pnas.1616926114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Betancur RR, Wiley EO, Arratia G, Acero A, Bailly N, Miya M, Lecointre G, Ortí G. 2017. Phylogenetic classification of bony fishes. BMC Evol. Biol. 17, 162 ( 10.1186/s12862-017-0958-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article and the codes for simulations can be found in the electronic supplementary material.