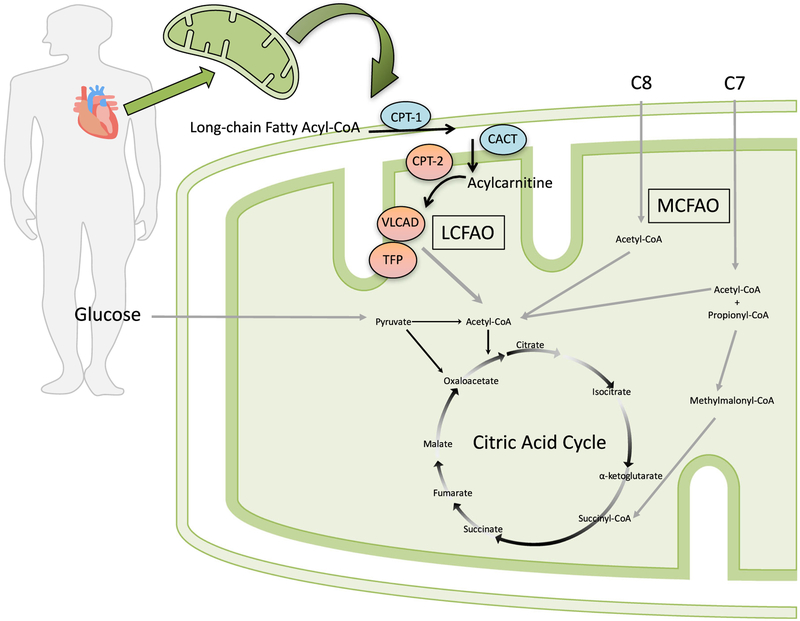
Fig. 1.
Model for the proposed benefit of triheptanoin (C7) compared to trioctanoate (C8) among patients with long-chain fatty acid oxidation disorders (LC-FAODs). Trioctanoate provides three 8 carbon fatty acids (C8) that, once imported into the mitochondria, are oxidized to 4 acetyl- CoA molecules. Triheptanoate provides three 7 carbon fatty acids (C7) that, once imported into the mitochondria, are oxidized to produce 2 acetyl-CoA and one propionyl-CoA molecule. Propionyl-CoA is converted to D-methylmalonyl-CoA by mitochondrial propionyl-CoA carboxylase followed by conversion to succinyl-CoA by D- methylmalonyl-CoA isomerase and L-methylmalonyl-CoA mutase. Succinyl-CoA is an intermediate of the citric acid cycle (CAC) and can increase intermediate pool size of carbon substrates. LCFAO = long-chain fatty acid oxidation; MCFAO = medium-chain fatty acid oxidation; CPT- 1 = carnitine palmitoyltransferase 1; CACT = carnitine acylcarnitine translocase; CPT-2 = carnitine palmitoyltransferase 2; VLCAD = very- long-chain acylCoA dehydrogenase; TFP = trifunctional protein. Patients enrolled in this clinical trial had CPT2, VLCAD or TFP deficiency, including long-chain 3-hydroxy acylCoA dehydrogenase (LCHAD) deficiency, as indicated by red enzyme color