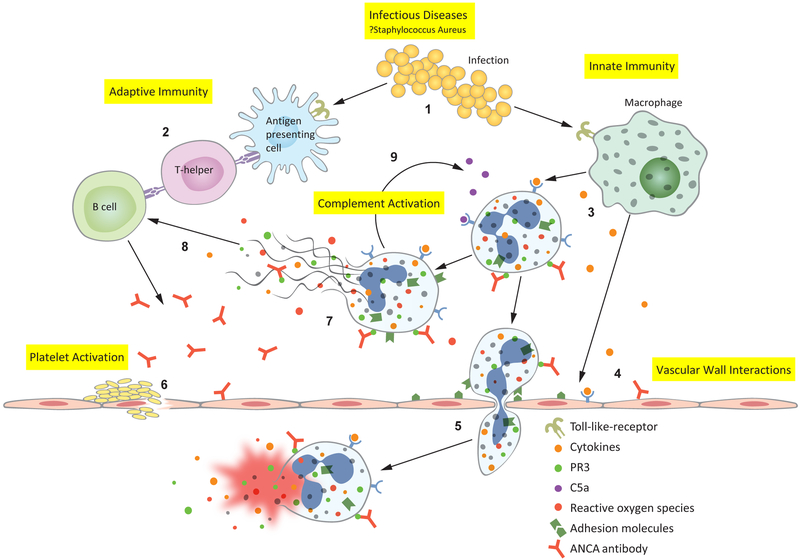
Figure 1:
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis pathophysiology revealed through gene expression pathways: 1. Infectious diseases, such as Staphylococcus aureus, activate Toll-like receptors (TLR) on antigen presenting cells and macrophages.(19) 2. Antimicrobial peptide homology with human antigens leads to the formation of pathogenic ANCAs.(20) 3. Macrophages secrete inflammatory cytokines which prime neutrophils— primed neutrophils express proteinase 3 (PR3) and myeloperoxidase (MPO) on their cell surface, allowing ANCA to bind and activate the neutrophil. 4. Cytokines and ANCAs activate endothelial cells, which express leukocyte adhesion molecules.(21-24) 5. ANCA-activated neutrophils migrate through the vascular wall where they degranulate, releasing reactive oxygen species and damage the vessel wall—thus causing the characteristic necrotizing vasculitis. 6. Platelets are activated at the sites of damaged endothelium, however their precise contribution to AAV pathophysiology is not well understood. 9. Activated neutrophils release neutrophil extracellular traps (NETs); NETs contain all of the intracellular contents of a neutrophil including MPO and PR3.(25) 8. Exposure of MPO and PR3 to the extracellular environment during NETosis drives further antibody formation. 9. Activated neutrophils also activate the alternative complement cascade, resulting in cleavage of C5 into C5a and C5b—C5a goes on to prime and attract more neutrophils leading to an amplification loop during active disease.(26)