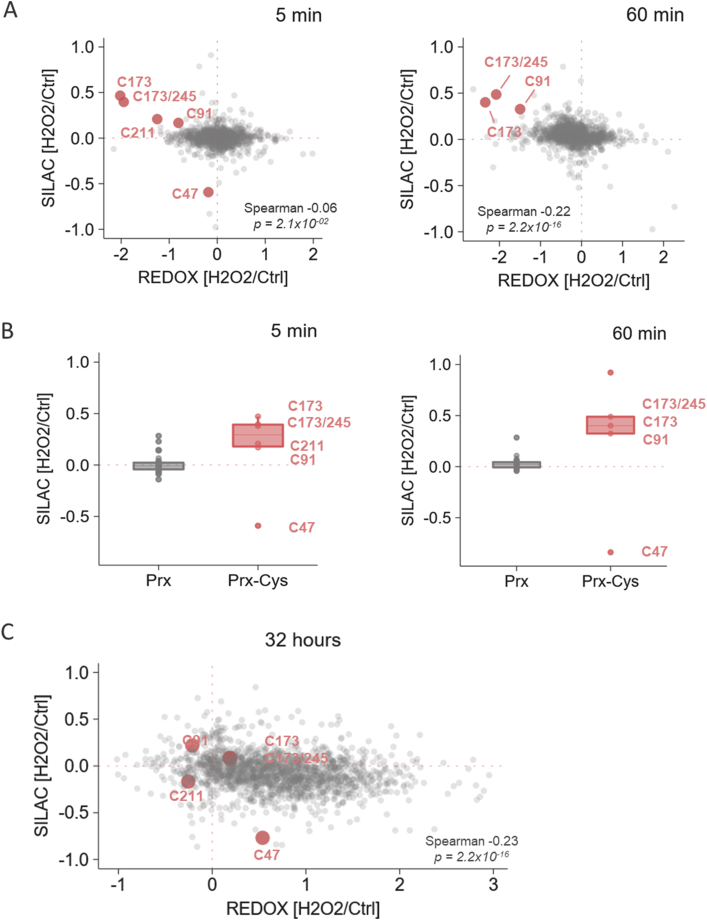
Fig. 3.
Cysteine reduction in peroxiredoxin peptides was related to higher intensity of H2O2-treated sample in SILAC channel. (A) Scatter plot of SILAC (y-axis) and ox/red (x-axis) ratio of successfully quantified cysteine-containing peptides in 5 and 60 min after H2O2-exposure. Significantly reduced peroxiredoxin cysteine-containing peptides were marked in red. Data were combined from two biological replicates. (B) H2O2/Ctrl SILAC ratio of peroxiredoxin peptides with the cysteine residue (Prx-Cys) and with the absence of cysteine (Prx) 5 and 60 min post H2O2-treatment. Intensity of cysteinyl Prx peptides was different in H2O2 and Ctrl SILAC channel in contrast to Prx peptides without cysteine, whose SILAC ratio is unchanged in both 5 and 60 min after H2O2-treatment. The peptide with peroxidatic C47 of Prx 6 had apparent decreased intensity in H2O2 SILAC channel compared to resolving cysteine residues listed in Table 1 (C) Scatter plot of SILAC ratio (y-axis) and relative oxidation (x-axis) of successfully quantified cysteine-containing peptides in 32-h sample. Peroxiredoxin cysteine-containing peptides significantly reduced in 5 and 60 min samples are marked in red. Data were combined from two biological replicates. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)