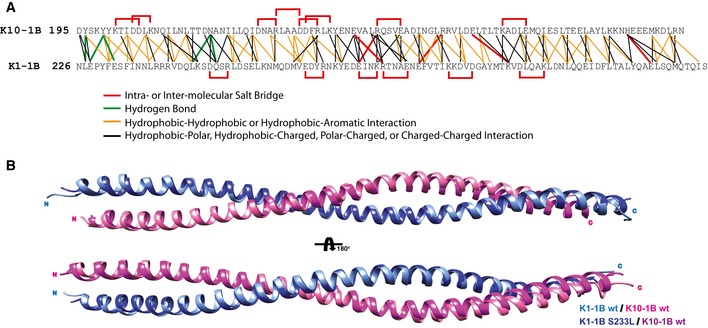
The amino acid contacts at the heterodimer interface for both wild‐type K1/K10‐1B and mutant K1S233L/K10‐1B X‐ray crystal structures were analyzed and plotted onto a single residue contact map. Intramolecular (within K1 or K10 only) and inter‐molecular (between K1 and K10) salt bridges are plotted red. Hydrogen bonds are plotted green. Interactions between hydrophobic residues are plotted orange (hydrophobic residues are defined as A, I, L, F, V, P, M, W), including hydrophobic interaction with the aromatic residue tyrosine. Other types of molecular contacts are plotted black. Analyses were performed using WHAT IF (defines atoms as “in contact” when the distance between their van der Waals surfaces is < 1.0 Å), ESBRI, and PDBePISA. Since S233K1 is a surface‐exposed residue, its mutation to L233 does not impact the heterodimer interface. Hence, the analysis of both heterodimer interfaces was used to obtain the contact map.