Abstract
Evidence suggests that exercise can modulate neuroinflammation and neuronal damage. We evaluated if such effects of exercise can be detected with positron emission tomography (PET) in a rat model of Parkinson’s disease (PD). Rats were unilaterally injected in the striatum with 6-hydroxydopamine (PD rats) or saline (controls) and either remained sedentary (SED) or were forced to exercise three times per week for 40 min (EX). Motor and cognitive functions were evaluated by the open field, novel object recognition, and cylinder tests. At baseline, day 10 and 30, glial activation and dopamine synthesis were assessed by [11C]PBR28 and [18F]FDOPA PET, respectively. PET data were confirmed by immunohistochemical analysis of microglial (Iba-1) / astrocyte (GFAP) activation and tyrosine hydroxylase (TH). [11C]PBR28 PET showed increased glial activation in striatum and hippocampus of PD rats at day 10, which had resolved at day 30. Exercise completely suppressed glial activation. Imaging results correlated well with post-mortem Iba-1 staining, but not with GFAP staining. [18F]FDOPA PET, TH staining and behavioral tests indicate that 6-OHDA caused damage to dopaminergic neurons, which was partially prevented by exercise. These results show that exercise can modulate toxin-induced glial activation and neuronal damage, which can be monitored noninvasively by PET.
Keywords: Dopamine synthesis, glial activation, positron emission tomography, Parkinson’s disease, exercise
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease among elderly and affects approximately 30 million people worldwide.1 PD patients account for a large portion of healthcare expenditures and represents a huge social and economic burden.2,3 Thus, adequate prevention and treatment strategies for PD are required.
One possible strategy to delay the onset and progression of PD is regular exercise since exercise was found to be inversely related to the incidence of neurodegenerative diseases.4,5 Exercise is a cheap and widely practiced activity that stimulates the molecular and cellular cascades that support and maintain brain plasticity.6,7 Intermittent exercise can lead to improvement of the dopaminergic system and recovery of motor behavior in an animal model of PD.8 Exercise induces an increase in the neurotrophic factors, that play an important role in central nervous system protection and recovery.5,9,10 An increase in neurotrophic factors in the nigrostriatal system can improve mitochondrial function and protect neurons from the detrimental effects of a neurotoxin.11 Besides neurotrophic factors, vascular endothelial growth factor and insulin growth factor 1 can reduce the levels of pro-apoptotic proteins and thus seem to indirectly participate in neuroprotection.5,7 This suggests that exercise may reduce the risk of developing neurodegenerative disorders and delay disease progression.5 To enable monitoring of the protective effects of exercise on brain functions in longitudinal study designs and to facilitate the translation of preclinical results to humans, noninvasive functional imaging tools would be desirable.
Positron emission tomography (PET) is an attractive tool for acquiring functional information on pathological processes in the brain. 6-[18F]Fluoro-L-3,4-dihydroxyphenylalanine ([18F]FDOPA) has been used as a PET tracer to monitor aromatic L-amino acid decarboxylase (AADC) activity in dopaminergic neurons. Since AADC is responsible for the conversion of DOPA into dopamine, [18F]FDOPA PET has been used as surrogate marker for the dopamine synthesis rate and presynaptic dopaminergic neuronal integrity. A decline in tracer uptake in striatum correlates with the severity of dopaminergic dysfunction.12 [18F]FDOPA PET enables the detection of presynaptic dopaminergic deficits in PD patients with excellent sensitivity and specificity, even in the early phases of the disease.13 Thus, [18F]FDOPA PET could be a useful tool to evaluate the efficacy of novel treatment strategies for PD, like exercise.14,15
Neuroinflammation can be detected in PD patients before a decrease in dopaminergic function can be observed with [18F]FDOPA PET.16 Glial activation is an important hallmark of neuroinflammation. Upon activation, microglia and astrocytes increase the expression of the 18kD-translocator protein (TSPO) in the outer mitochondrial membrane.17–20 Overexpression of TSPO can be detected by PET with a suitable tracer. The TSPO ligand [11C]-(R)-PK11195 has been most frequently used to evaluate glial activation. However, this PET tracer has some limitations21,22 and therefore second generation of TSPO PET tracers has been developed. [11C]PBR28 is a second generation TSPO tracer that has been applied successfully in both preclinical and clinical studies.17,21–23
In the present study, we aimed to investigate the beneficial effects of exercise on glial activation and dopaminergic system degeneration in a PD model, using [11C]PBR28 and [18F]FDOPA PET, respectively. Postmortem immunohistochemical analysis was performed to confirm PET imaging results. As an animal model for PD, rats were treated by unilateral striatal injection of 6-hydroxydopamine (6-OHDA). Motor and cognitive functions were assessed in behavioral tests and correlated to imaging data.
Material and methods
Experimental animals
Animal experiments were carried out according to the Dutch Regulations for Animal Welfare. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Groningen (DEC 6689F) and reported in compliance with ARRIVE guidelines. Outbred male Hannover-Wistar rats (three months, n = 44) were purchased from Janvier labs (France) and group-housed in thermo-regulated (21 ± 2℃) and humidity-controlled rooms, under an inverted 12–12-h light-dark cycle (lights on at 12 PM). Food and water were available ad libitum. The rats were allowed to acclimatize for at least seven days. Animals were fed with standard laboratory chow (RM1. Special diets services, UK). Animals were randomly divided into 4 groups of 11 animals each: (1) sedentary control animals (SED), which were injected with vehicle into the right striatum; (2) exercised control animals (EX), which were injected with vehicle into the right striatum and were forced to exercise from day 2 after the surgery until the end of the experiment; (3) sedentary PD animals (SED + PD), which were injected with 6-OHDA into the right striatum; and (4) exercised PD animals (EX + PD), which were injected with 6-OHDA into right striatum and were forced to exercise from day 2 after the surgery until the end of the experiment. For each group, eight animals per group were assigned to the PET procedures and three animals were terminated on day 10 for immunohistochemistry. Sample size calculation was performed with G*Power 3.1.9.2 software (Universität Düsseldorf, Germany) based on published data for [18F]FDOPA PET15 and tyrosine hydroxylase (TH) expression24 in PD rats. We expected exercise to have a smaller effect than 6-OHDA injection. Assuming an effect size of 1.7 (power 80%; alpha 0.05), the sample size was estimated to be 8. Sample size calculation for Iba-1 and GFAP immunohistochemistry at day 10 was based on published data.24 Assuming an effect size of 3.36 (power 80%; alpha 0.05), the sample size was estimated to be 3. Figure 1 summarizes the experimental design. Two animals died during surgery (both SED group) and one died during the PET scan at day 10 (EX group). Data from some animals were lost due to methodological issues. The numbers of animals included in the analysis for each experiment are indicated in the tables in the Supplementary data. Investigators were not blinded for group allocation, since PET imaging analysis was fully automated and thus operator-independent.
Figure 1.
Experimental design. Sedentary controls (SED); Exercised controls (EX); Sedentary PD rats (SED + PD); and exercised PD rats (EX + PD). Open field test (OFT); Novel object recognition (NOR); short-term memory (SM); long-term memory (LM).
Surgical procedure
The animals were anesthetized with isoflurane mixed with oxygen (5% induction, 2% maintenance, 0.8 L/min) and placed in a stereotaxic apparatus (Kopf instruments, Germany). After craniotomy, two 0.5 µL aliquots of 3 µg 6-OHDA hydrochloride (H4381, Sigma) and 0.3% ascorbic acid in saline (PD animals) or 0.3% ascorbic acid in saline (control animals) were injected with a microinjection pump (CMA 100, CMA Microdialysis AB, Sweden) over a period of 5 min each. The solutions were injected into the right striatum at the following coordinates: (1) AP: +1.12, L: 2.6, V: 5 mm; (2) AP: +0.2, L: 3.0, V: 4.5 mm relative to Bregma and ventral to the dura mater.25 After infusion, the syringe needle (Hamilton 51315-02) was left in the infused region for 3 min to avoid reflux of the solution. The incision was sutured and the animals were kept isolated in cages for two days. To reduce discomfort, pain medication (flunixin-meglumin – 1 mg/kg, s.c.) was given before surgery and at 24 h after surgery. If the rats still showed signs of discomfort after 48 h, an extra dose of analgesic was given.
Exercise
Rats were forced to exercise in a motorized running wheel for three days per week, starting two days after surgery. All animals, including animals from the sedentary groups, were adapted to the motorized running wheel (TSE systems, 303400 series, 252 mm diameter) between baseline measurements and surgery by forcing them to run for 15 min on two consecutive days (6.7 m/min). Exercised animals were subjected to a moderate exercise protocol at a speed of 10 m/min for 40 min (13 revolutions/min, approximately 400 m/day).8,26 SED and SED + PD animals were placed in cages near the running wheel to become familiarized with the novel surrounding.
Open field test
Animals were individually submitted to the open field arena (100 × 100 × 40 cm) for 10 min before the baseline PET scan (6 days before surgery) and 28 days after surgery. The parameters analyzed included total distance moved and speed.27,28 The open field arena was cleaned with 70% ethanol before each behavioral test to eliminate possible bias due to odors left by previous rats.29 The behavioral tests were video-recorded and analyzed using the Ethovision XT8.5 software (Noldus Information Technology, The Netherlands).
Novel object recognition
NOR is a test that analyzes both short-term and long-term working memory, depending on the interval between tests.30 The experiment was carried out at baseline and 28 days after surgery. All objects used for the test had similar textures, colors, and sizes, but distinctive shapes. The rats were placed in a square black arena (50 × 50 × 40 cm) containing two identical objects (A1 + A2) for the habituation and training phase, as previously described,31 but with some modifications.27 After 5 min, the rats were removed from the arena and placed into their home cage. After 1 h (short-term memory, SM)32 and 24 h (long-term memory, LM), the rats were placed in the arena again for 5 min, with one objects replaced by a novel object (SM: B + A2, LM: C + A2). The 28-day test was carried out with new objects (D1 + D2, E + D2, F + D2). The behavioral tests were video-recorded for further analysis. Exploration of an object is defined as the time the animal spends with its head oriented towards the object, within two centimeters from the object (sniffing). The discrimination index was calculated by: [(time exploring the new object)/(total time exploring the two objects)] × 100%.27,33
Cylinder test
The cylinder test is a test for unilateral deficits in voluntary forelimb use.27,34 The cylinder test was performed at baseline, day 9 and 29 after surgery. Rats were placed in a transparent cylinder (diameter: 20 cm, height: 30 cm) for 5 min, with a mirror located behind the cylinder to allow a 360° vision.35 The number of forepaw contacts to the cylinder wall was counted.35,36 The cylinder test was scored as the contralateral bias: [(the number of contralateral limb contact)/(number of total limbs contacts)] × 100%.27,35 Healthy rats should score on average 50% in this test.37
Immunohistochemistry
On day 10 and 30 after surgery, animals were anesthetized with ketamine (60 mg/kg i.p.) and medetomidine (0.4 mg/kg i.p.), and perfused through the left ventricle with 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4). Anti-TH (mouse monoclonal, Millipore MAB5280), anti-ionized calcium-binding adapter molecule 1 (Iba-1, microglia marker) (rabbit polyclonal, Wako laboratory Chemicals 019-19741), and anti-glial fibrillary acidic protein (GFAP, astrocyte marker) (mouse monoclonal, Sigma-Aldrich G3893) antibodies were used. The secondary antibody was a biotinylated donkey anti-mouse IgG for TH and GFAP (715-065-151, Jackson Laboratories), and a biotinylated goat anti-rabbit IgG for Iba1 (111-065-003, Jackson Laboratories). The protocol was the same as used previously.8,26 Coronal brain sections (30 µm) were incubated overnight at 22℃ with a primary antibody solution containing 5% normal donkey serum (for TH and GFAP), or normal goat serum (for Iba-1) in 0.3% Triton X-100 in PBS diluted 1:1000. Sections were subsequently incubated with a 1:200 diluted secondary antibody solution for 2 h. The sections were processed with the ABC Elite kit (Vector Labs, USA) for 2 h and the labeling was developed with 0.05% diaminobenzidine tetrahydrochloride and 0.03% hydrogen peroxide in PBS.
Immunohistochemistry analysis
Digital images of five stained sections (150 µm between sections) of striatum, SNc and hippocampus were acquired from each rat using a microscope and digital camera. A stereotaxic atlas was used as an anatomical reference.25 The integrated density of Iba-1 and GFAP staining was measured with the Imaging J threshold plugin (Image J, NIH/USA). For TH staining in SNc, the number of positive cells was also counted with the cell counter plugin and represented as positive cells per mm2. For analysis of TH staining in striatum, three areas of 0.0044 mm2 for each slice were selected to avoid regions of fiber bundles. For other regions and antibodies, random areas of 0.18 mm2 were selected. To avoid inclusion of the mechanical damage by the injection in the analysis, only slices at a distance of at least 50 µm from the injection site were analyzed. Optical density for right and left hemispheres was assessed separately and as experimental-to-control hemisphere ratios.8
PET imaging
PET scans were acquired at baseline and at day 10 and 30 after surgery using a small-animal PET camera (Focus 220, Siemens Medical Solutions, USA). [11C]PBR28 PET was performed in the morning, and [18F]FDOPA PET in the afternoon, with at least 3-h interval. Two rats from different experimental groups were scanned simultaneously in each scanning session. Rats were anesthetized with isoflurane mixed with oxygen (5% induction, 2% maintenance, 0.8 L/min). Thirty minutes before [18F]FDOPA injection, the peripherally acting AADC inhibitor, benserazide hydrochloride (20 mg/kg, 10 mg/mL in PBS, AvaChem Scientific, New Jersey, USA), and the catechol-O-methyl transferase inhibitor, Entacapone (20 mg/kg, 50 mg/mL in DMSO, AvaChem Scientific, New Jersey, USA) were administered i.p. to reduce tracer metabolism.14,15 Rats were positioned in the camera in a transaxial position with their heads in the center of the field of view. A 10-min transmission scan with a 57Co point source was performed to correct for attenuation, scatter and random coincidences during image reconstruction. The animals were injected with 48 ± 22 MBq [11C]PBR28 or 25 ± 3 MBq [18F]FDOPA via the penile vein. The injected amount of [11C]PBR28 (0.86 ± 0.70 nmol, p = 0.98) and [18F]FDOPA (304 ± 147 nmol, p = 0.99) was not significantly different between groups and time points. After a tracer distribution time of 40 min for [11C]PBR28 or 60 min for [18F]FDOPA, a 30-min static emission scan was acquired. The body temperature of the rats was maintained with heating pads, eye lubricant was applied onto the eyes to prevent dehydration, and heart rate and blood oxygen levels were monitored with a BioVet system (M2M Imaging, USA). After the scans, the rats were either allowed to recover in their home cages or terminated for immunohistochemistry.
PET image reconstruction and analysis
Emission sinograms were iteratively reconstructed into a single frame of 30 min (OSEM 2D; 4 iterations and 16 subsets), after being normalized and corrected for attenuation, scatter, and decay of radioactivity. PET image processing was performed with PMOD 3.7 software (PMOD Technologies Ltd, Switzerland). Scans were automatically registered to tracer-specific PET templates38 constructed from the baseline scans acquired in this study. Volumes of interest (VOI) were constructed based on previously defined templates.38 Separate VOIs for left and right striatum, midbrain and hippocampus were used for [11C]PBR28 PET analysis. VOIs for striatum and cerebellum were constructed for [18F]FDOPA PET analysis. The brain radioactivity concentration was calculated in each VOI and expressed as standardized uptake value (SUV): [tissue activity concentration (MBq/g) × body weight (g)]/[injected dose (MBq)]. A tissue density of 1 g/ml was assumed. The imaging data were expressed as tracer uptake in the experimental and control hemisphere separately and as the ratio between hemispheres. [18F]FDOPA uptake was normalized to the cerebellum uptake (striatal uptake divided by the uptake in cerebellum)39,40 to correct for metabolite accumulation inside the brain.39 Cerebellum was considered a reference region devoid of dopamine synthesis. [11C]PBR28 data were not normalized to a reference region, because there is no brain region devoid of TSPO receptors.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Statistical analyses of baseline data from behavioral tests and PET imaging were performed using one-way ANOVA. After surgery, data were analyzed using two-way ANOVA, followed by a Tukey posthoc correction for multiple comparisons whenever appropriate. ‘Exercise’ and ‘surgery’ were used as the variables between groups. Statistical analysis of changes in body weight was performed using the generalized estimating equations (GEE) model with independent correlation matrix to account for repeated measurements and missing data in the longitudinal design.41 The variables ‘time point’, ‘group’ and the interaction ‘time point’ × ’group’ were included in a factorial design. Differences were considered statistically significant when p < 0.05. The correlation between TH staining and [18F]FDOPA uptake was analyzed with a linear regression method. Within-group comparison between baseline and post-surgery data was performed by a paired Student’s t-test. All data were analyzed using IBM SPSS Statistics 24 (SPSS Inc., USA).
Results
Body weight
No significant differences in body weight between groups [F(3,40) = 0.31; p = 0.82] were observed at baseline (Figure 2(a), Supplementary Table 1). 6-OHDA injection significantly decreased body weight in the first week after surgery (SED vs. SED + PD p < 0.0001; EX vs. EX + PD p < 0.01). All groups significantly gained body weight from the second week after surgery onward. Body weight increased about 22 g/week in sedentary animals (p < 0.0001) and only about 14 g/week in exercised animals (p < 0.0001). Consequently, EX + PD animals were significantly lighter at the end of the experiment than SED + PD animals (ca. 8%, p < 0.05).
Figure 2.
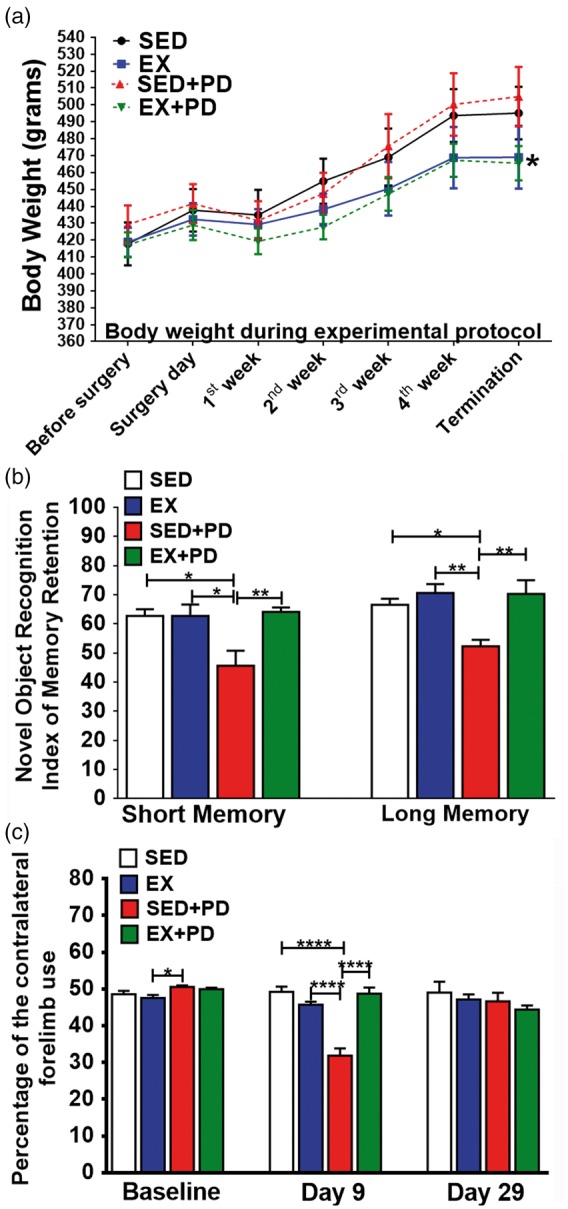
(a) Body weight of the animals throughout the experimental protocol. SED + PD vs. EX + PD (*p < 0.05). (b) Results of the novel object recognition test for short-term (left) and long-term (right) memory 28/29 days after surgery. Results are expressed as index of memory retention. (c) Cylinder test data, showing the percentage of the contralateral forelimb use at baseline, 9 days and 29 days after the surgery. Sedentary controls (SED); Exercised controls (EX); Sedentary PD rats (SED + PD); and exercised PD rats (EX + PD). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Open field
At baseline, no significant differences in distance traveled and average speed between groups were observed [(Distance: F(3,31) = 0.22, p = 0.88); Speed: F(3,31) = 0.75, p = 0.53)]. At day 28, animals from all groups showed a decrease in the distance traveled and average speed, when compared to baseline (ca. 50%, p < 0.001). However, no significant differences between experimental groups were observed at day 28 [(Distance: F(1,25) = 0.23, p = 0.63); Speed: F(1,25) = 0.46, p = 0.50)] (Supplementary Table 2).
Novel object recognition
When exposed to similar objects, no significant differences in the time spent exploring each object were observed, neither at baseline [F(3,32) = 0.83, p = 0.49] nor at day 28 [F(3,25) = 2.26, p = 0.11] (Supplementary data – Table 3). No between-group differences in short-term memory (F(3,32) = 0.51, p = 0.68) and long-term memory (F(3,32) = 2.63, p = 0.07) were found at the baseline. Within-group comparison of short-term memory showed that the memory index of SED + PD animals was significantly reduced between baseline and day 28 (−23%, p < 0.01), whereas the long-term index of memory retention was also decreased (−27%, p < 0.0001). Other groups did not show any significant differences in short-term or long-term memory between baseline and day 28. As a result, between-group comparison at day 28 after surgery (Figure 2(b)) showed a significantly lower (ca. 28%, F(1,25) = 7.02, p = 0.01) short-term index of memory retention for SED + PD animals than for other groups (SED p < 0.05; EX + PD p < 0.01). Long-term memory of the SED + PD group also showed a significantly lower effect for lesion [F(1,25) = 4.61, p = 0.04] and exercise [F(1,25) = 10.58, p = 0.003], when compared to other groups (ca. 25%, SED p < 0.05; EX and EX + PD p < 0.01).
Cylinder test
At baseline, asymmetric forelimb use was not significantly different between groups [F(3,40) = 2.40; p = 0.08]. At day 9, the SED + PD group showed significantly lower contralateral forelimb use than other groups (SED 35%, EX 30%, EX + PD 35%, p < 0.0001) [F(1,38) = 42.24; p < 0.0001]. At day 29, no significant differences between groups were observed anymore [F(1,25) = 0.01; p = 0.92] (Figure 2(c), Supplementary data – Table 4).
[11C]PBR28 PET
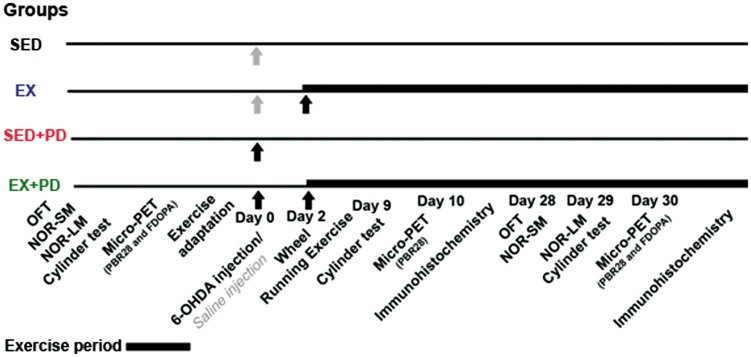
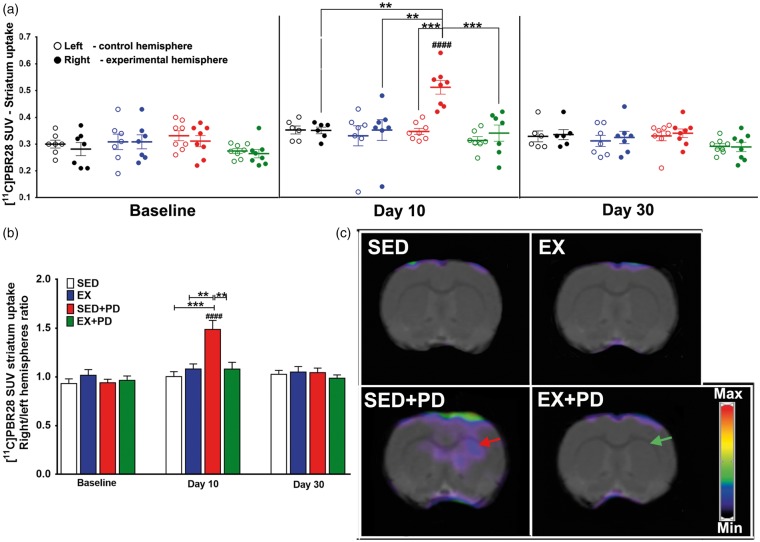
[11C]PBR28 uptake was analyzed for total midbrain, since the SNc could not be reliably delineated in the PET images due to the limited spatial resolution of the PET camera (1.4 mm). Tracer uptake in the affected midbrain did not significantly differ between groups at any time point (baseline [F(3,52) = 0.42; p = 0.74]; day 10 [F(3,48) = 0.499, p = 0.068]; day 30 [F(3,50) = 0.75, p = 0.52]) and neither did the ratio between the affected and control midbrain (baseline [F(3,26) = 0.88; p = 0.47]; day 10 [F(1,24) = 1.44, p = 0.24]; day 30 [F(1,25) = 0.11, p = 0.74]) (Supplementary data – Table 5). [11C]PBR28 uptake in the affected striatum of the SED + PD group was significantly increased at day 10 (F(3,48) = 4.68, p = 0.006), but not at baseline (F(3,52) = 2.049; p = 0.12) or day 30 (F(3,50) = 0.55, p = 0.65). Consequently, the uptake ratio between hemispheres in the SED + PD group at day 10 was significantly higher when compared to baseline (60%, p < 0.0001) and to the striatal uptake ratio in other groups at day 10 (ca. 49%, p < 0.01F(1,24) = 7.55, p = 0.01). At day 30, no significant differences in striatal uptake between hemispheres or between groups were observed anymore [F(1,25) = 2.46; p = 0.13] (Figure 3). In hippocampus, there was no significant difference in tracer uptake between hemispheres or between groups at baseline [F(3,26) = 0.62; p = 0.61]. At day 10, SED + PD animals showed significant effects for 6-OHDA injection [F(1,26) = 5.50; p = 0.03] and for exercise [F(1,26) = 5.74; p = 0.03]. In particular, [11C]PBR28 uptake in the affected hippocampus of SED + PD rats was significantly increased when compared to baseline and to other groups at day 10 (ca. 30%, p < 0.001) [F(1,26) = 7.55, p = 0.01]. No significant differences in hippocampal [11C]PBR28 uptake were observed anymore at day 30 [F(1,25) = 2.16; p = 0.15] (Figure 4) (Supplementary data – Table 5).
Figure 3.
Results of [11C]PBR28 PET at baseline, 10 days and 30 days after surgery. (a) Tracer uptake in the experimental (right) and control (left) striatum, expressed as Standard Uptake Value (SUV). (b) Tracer uptake ratio between experimental and control striatum. (c) Examples of transaxial [11C]PBR28 PET images of the striatal region acquired at day 10 after surgery. The hemisphere of sedentary animals in which 6-OHDA was injected shows increased [11C]PBR28 uptake (red arrow). Note that exercise reversed this effect, resulting in a normalization of [11C]PBR28 uptake in the EX + PD group (green arrow). Sedentary controls (SED); Exercised controls (EX); Sedentary PD rats (SED + PD); and exercised PD rats (EX + PD). Standard uptake value (SUV). Statistical differences between groups for the same brain hemisphere and between hemispheres for same group are presented as *p < 0.05; **p < 0.01; ***p < 0.001; statistical differences between baseline and day 10 and 30 are indicated by #p < 0.05; ##p < 0.01; ####p < 0.0001.
Figure 4.
Results of [11C]PBR28 PET at baseline, 10 days and 30 days after surgery. (a) Tracer uptake in the experimental (right) and control (left) hippocampus, expressed as Standard Uptake Value (SUV). (b) Tracer uptake ratio between experimental and control hippocampus. (c) Examples of transaxial [11C]PBR28 PET images of the hippocampus region acquired at day 10 after surgery. The hemisphere of sedentary animals in which 6-OHDA was injected shows increased [11C]PBR28 uptake (red arrow). Note that exercise reversed this effect, resulting in a normalization of [11C]PBR28 uptake in the EX + PD group (green arrow). Sedentary controls (SED); Exercised controls (EX); Sedentary PD rats (SED + PD); and exercised PD rats (EX + PD). Standard Uptake Value (SUV). Statistical differences between groups for the same brain hemisphere and between hemispheres for same group are presented as *p < 0.05; **p < 0.01; ***p < 0.001; ***p < 0.0001. Statistical differences between baseline and day 10 and 30 are indicated by ###p < 0.001.
Ionized calcium-binding adapter molecule 1
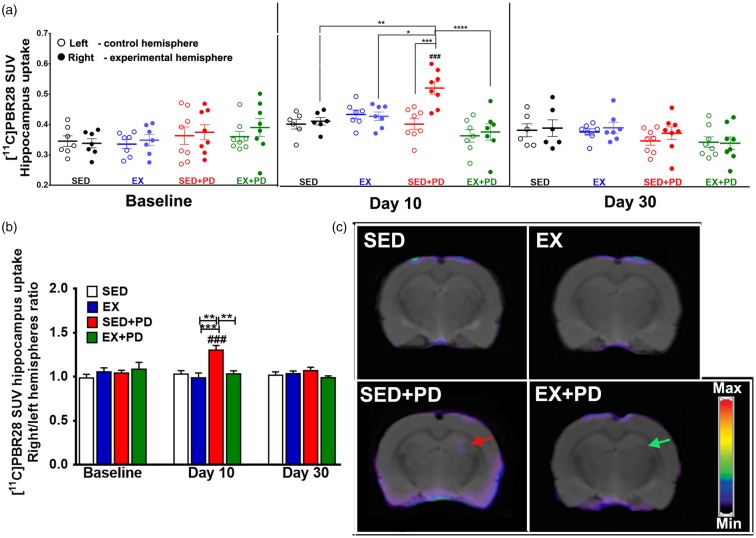
Iba-1 staining was performed on SNc, striatum, and hippocampus sections (Figure 5(a) to (c)). Injection of 6-OHDA caused a significant increase in Iba-1 staining in all analyzed regions at day 10 after surgery (SED + PD vs. SED: SNc + 120%; p < 0.001; striatum + 94%, p < 0.001; hippocampus + 164%, p < 0.01). Exercise completely suppressed the 6-OHDA-induced activation of microglia at day 10, as Iba-1 staining in all investigated brain regions of the EX + PD group was reduced to the level of exercised controls (EX + PD vs. SED + PD: SNc − 53%, p < 0.01; striatum − 79%, p < 0.001; hippocampus − 178%, p < 0.01) (SNc [F(1,8) = 33.35, p = 0.0004]; striatum [F(1,8) = 29.37, p = 0.0006]; hippocampus [F(1,8) = 17.71, p = 0.003]). At day 30, microglial activation was still increased in SNc (EX + PD vs. SED + PD p = 0.71; SED + PD vs. SED:+56%, p < 0.0001; EX + PD vs. EX: + 68%, p < 0.0001) [F(1,25) = 59.17, p < 0.0001]. In contrast, Iba-1 staining in striatum and hippocampus of EX + PD rats was completely normalized to the level of sedentary controls (striatum [F(1,25) = 3.66, p = 0.07]; hippocampus [F(1,25) = 2.29, p = 0.14]). Exercise caused a small but significant reduction in Iba-1 staining in striatum of 6-OHDA-treated animals at day 30 (EX + PD vs. SED + PD: −17%, p < 0.05), despite Iba-1 staining in SED + PD animals not being significantly different from sedentary controls (Supplementary data – Figure S1, Table 5).
Figure 5.
Iba-1 immunostaining of activated microglia 10 days and 30 days after the surgery in the experimental (right) and control (left) substantia nigra (a), striatum (b) and hippocampus (c). Note the peak of microglial activation in the SED + PD animals at 10 days after the surgery for all the structures analyzed. At day 30, both groups of PD rats still revealed an increase in the microglial activation in the substantia nigra. Statistical differences between groups for the same brain hemisphere and between hemispheres for same group are presented as *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
GFAP immunostaining of astrocytes 10 days and 30 days after the surgery in the experimental (right) and control (left) substantia nigra (d), in the striatum (e) and in the hippocampus (f). Note the peak of astrocyte activation in the striatum of PD rats at 10 days after the surgery, which normalized to control levels at 30 days in the exercised PD rats. Statistical differences between groups for the same brain hemisphere and between hemispheres for same group are presented as *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Glial fibrillary acidic protein
6-OHDA injection or exercise did not have any significant effect on GFAP optical density in the SNc or hippocampus at day 10 (SNc: [F(1,8) = 3.55, p = 0.10]; hippocampus: [F(1,8) = 0.23, p = 0.65]) or 30 after surgery (SNc: [F(1,25) = 0.31, p = 0.58]; hippocampus: [F(1,25) = 0.42, p = 0.52]). On the other hand, 6-OHDA injection caused a significant increase in astrocyte staining in striatum at day 10 after surgery (SED + PD vs. SED + 164%, p < 0.01). Exercise could not suppress this effect (EX-PD vs. SED-PD p = 0.92; EX + PD vs. SED + 141%, p < 0.05) [F(1,8) = 25.90, p = 0.0009]. At day 30, the 6-OHDA effect on astrocyte activation in striatum had not completely disappeared. Instead, GFAP staining in striatum was significantly reduced in exercised PD rats (EX-PD vs. SED-PD −30%, p < 0.05) with significant effects for 6-OHDA [F(1,25) = 7.44, p = 0.01] and for exercise [F(1,25) = 9.23, p = 0.005] (Figure 5(d) to (f), Supplementary data – Figure S2, Table 5).
[18F]FDOPA PET
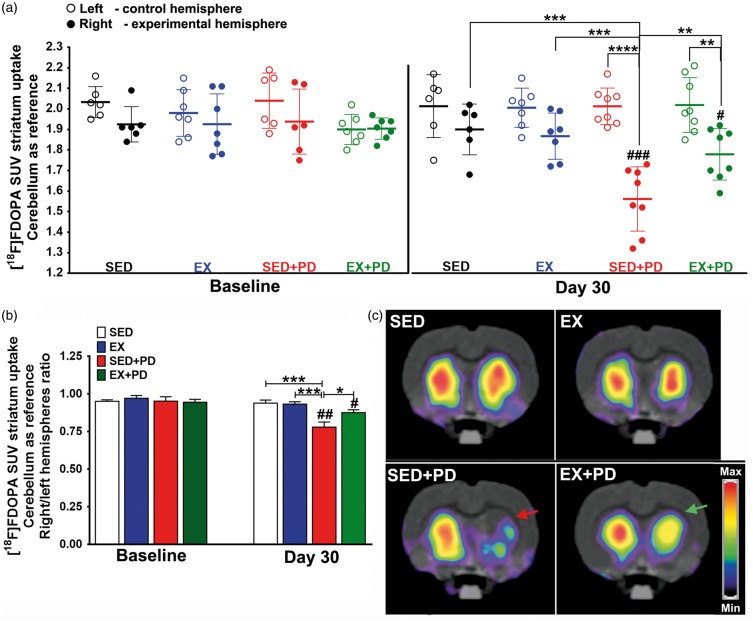
[18F]FDOPA uptake in striatum did not show any significant differences between hemispheres or between groups at baseline [F(3,22) = 0.34; p = 0.79]. Fours animals (one from each group) did not undergo a baseline [18F]FDOPA scan, due to tracer production failures. The within-group comparison showed that 6-OHDA injection caused a significant reduction in [18F]FDOPA uptake in the affected striatum of sedentary PD rats between baseline and day 30 (−18%, p < 0.01). Exercised PD animals showed a substantially smaller reduction in [18F]FDOPA uptake between baseline and day 30 (−6%, p < 0.05), suggesting exercise could partially protect the dopaminergic system. Between-group comparison at day 30, confirmed that sedentary PD rats had significantly lower [18F]FDOPA uptake in the affected striatum than sedentary controls (SED + PD vs. SED − 17%, p < 0.001). In contrast, [18F]FDOPA uptake in exercised PD rats at day 30 was not significantly different from controls (EX + PD vs. EX p = 0.36; EX + PD vs. SED p = 0.30) and significantly higher than in sedentary PD rats (EX + PD vs. SED + PD + 12%, p < 0.05) [F(1,25) = 4.43; p = 0.04] (Figure 6) (Supplementary data – Table 6).
Figure 6.
Results of [18F] FDOPA PET at baseline and 30 days after surgery. (a) Tracer uptake in the experimental (right) and control (left) striatum is normalized to the uptake in cerebellum and expressed as Standard Uptake Value (SUV) (b) Tracer uptake ratio between right and left striatum. (c) Examples of [18F]FDOPA PET images of transaxial brain sections containing the striatum, acquired at day 30 after surgery. [18F]FDOPA uptake is reduced in the striatum injected with 6-OHDA in sedentary animals (red arrow). Note that exercise partly reversed this effect in EX + PD animals (green arrow). Sedentary controls (SED); Exercised controls (EX); Sedentary PD rats (SED + PD); and exercised PD rats (EX + PD). Statistical differences between groups for the same brain hemisphere and between hemispheres for same group are presented as *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Statistical differences in tracer uptake between baseline and day 30 are indicated as #p < 0.05; ## p < 0.01; ###p < 0.001.
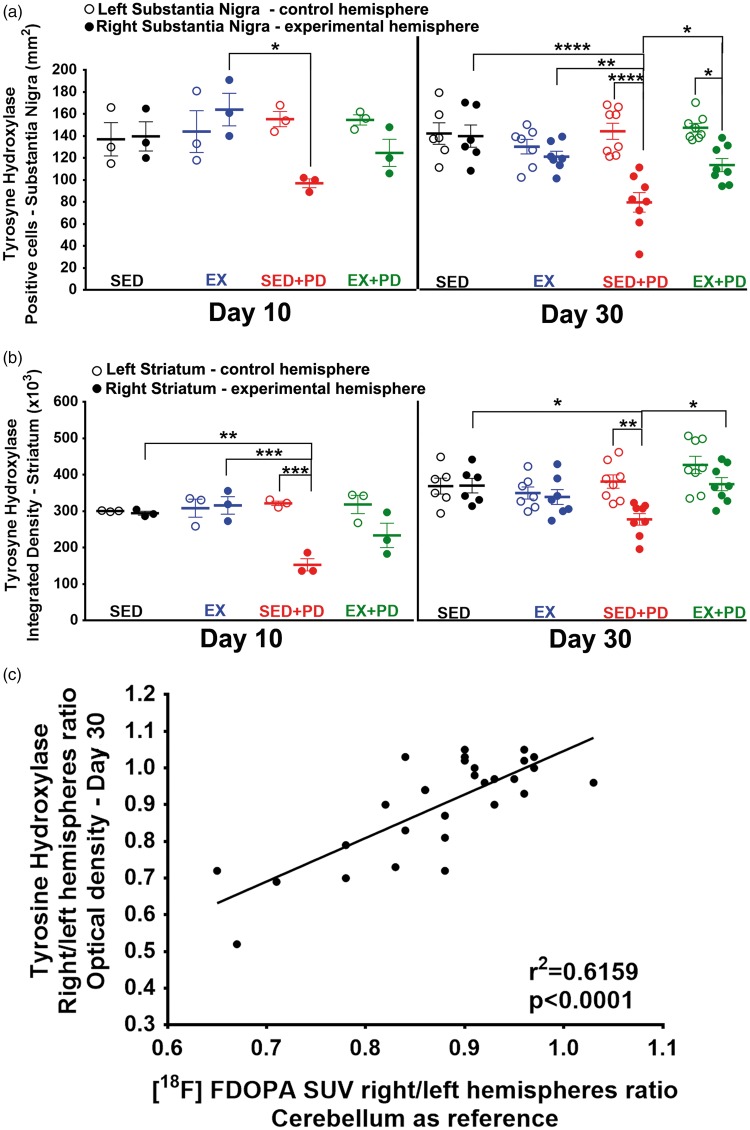
TH
Injection of 6-OHDA caused a significant decrease in the percentage of TH-positive cells in the SNc (Figure 7(a)) of both sedentary (SED-PD vs. SED − 42%, p < 0.001) and exercised PD rats (EX + PD vs. EX − 38%, p < 0.01) at day 10 after surgery [F(1,8) = 62.38, p < 0.0001]. At day 30, the percentage of TH-positive cells in SNc was still significantly reduced in sedentary PD rats (SED + PD vs. SED − 46%, p < 0.001). In exercised PD rats, however, the percentage of TH-positive cells was not significantly different from exercised controls (EX + PD vs. EX p = 0.06), and significantly higher than in sedentary PD rats (EX + PD vs. SED + PD 41%, p = 0.05) [F(1,25) = 31.31, p < 0.0001]. TH staining in striatum at day 10 (Figure 7(b)) was also significantly lower in 6-OHDA-injected animals than in saline controls (SED + PD vs. SED − 51%, p < 0.01; EX + PD vs. EX − 31%, p < 0.05). Although the reduction in TH staining was substantially smaller in exercised than in sedentary PD rats, this difference was not statistically significant (EX + PD vs. SED + PD 47%, p = 0.09), indicating no interaction between factors but significance for 6-OHDA [F(1,8) = 52.47, p < 0.0001] and exercise [F(1,8) = 5.84, p = 0.04]. Thirty days after surgery, the sedentary PD group still showed significantly decreased TH staining in striatum (SED + PD vs. SED − 20%, p < 0.01). However, TH staining in striatum had completely normalized to control levels in exercised PD rats (EX + PD vs. EX p = 0.50), although the difference from sedentary PD rats was not significant yet (EX + PD vs. SED + PD 14%, p = 0.23) [F(1,25) = 12.53, p = 0.002]. Interestingly, the TH optical density staining ratio between the affected and contralateral striatum correlated well with the [18F]FDOPA uptake ratio (r2 = 0.62, p < 0.0001) (Figure 7(c)) (Supplementary data – Figure S3, Table 7).
Figure 7.
(a) The number of TH-positive cells per mm2 for right and left substantia nigra 10 days and 30 days after surgery; (b) TH optical density for in the affected (right) and contralateral (left) striatum 10 days and 30 days after surgery; (c) Correlation between [18F]FDOPA uptake (SUV) ratio and TH staining ratio for striatum at 30 days after surgery. Sedentary controls (SED); Exercised controls (EX); Sedentary PD rats (SED + PD); and exercised PD rats (EX + PD). Statistical differences between groups for the same brain hemisphere and between hemispheres for same group are presented as *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Discussion
The present study showed that exercise is able to suppress neurotoxin-induced glial activation, reduce degeneration of the dopaminergic system and normalize memory and motor deficits in a rat model of PD. Exercise has been suggested as a potential intervention in neurodegenerative diseases. However, its beneficial effects were only determined indirectly by assessment of clinical symptoms or postmortem tissue analysis. In this study, we directly demonstrated the effects of exercise on glial activation and the dopaminergic system in a noninvasive manner using PET. Imaging results were confirmed by postmortem immunohistochemical analysis.
Previous studies demonstrated that injection of 6-OHDA in striatum increases the expression of microglial markers and decreases TH expression.42 Despite the small sample size for immunohistochemistry on day 10, Iba-1 staining in this study confirmed that unilateral 6-OHDA injection in striatum causes robust microglial activation, not only in striatum, but also in SNc and hippocampus of sedentary PD rats. At day 30, activated microglia were still present in SNc, but not in striatum and hippocampus. [11C]PBR28 PET provided similar results as Iba-1 staining for striatum and hippocampus, but could not detect glial activation in SNc. This can be explained by the limited spatial resolution of the PET camera, which precluded analysis of small brain structures, like SNc, in rats. When the whole midbrain was analyzed instead, glial activation could not be detected since the specific signal derived from SNc was averaged out over a large volume (partial volume effect). 6-OHDA injection not only caused microglia activation, but also increased astrocyte density in the striatum of sedentary rats, but not in SNc or hippocampus. Sustained exercise was hypothesized to have anti-inflammatory properties that can counteract the effect of 6-OHDA. In this study, both Iba-1 staining and PET imaging showed that intermittently forced exercise could indeed suppress the activation of microglia in striatum, SNc and hippocampus. In line with our observation, previous studies described that exercise reduces the number of circulating pro-inflammatory monocytes and increases the number of regulatory T cells. In addition, levels of anti-inflammatory and neuroprotective molecules are enhanced in peripheral blood after physical activity.7 However, the mechanism through which exercise affects the immune system is not fully understood yet.
At day 30, microglia activation had spontaneously resolved in striatum and hippocampus, but not in SNc. Microglia activation in SNc seems to be associated with persistent dopaminergic injury. This observation is in line with other studies in animals and postmortem studies on brain tissue of PD patients.43 Attempts to elucidate how activated microglia contribute to neuronal loss in the SNc have generated conflicting results.43 In EX + PD animals, this persisting microglia activation appeared to have caused somewhat less degeneration of the dopaminergic system in SNc. Exercise may have changed the phenotype of microglia in SNc from the pro-inflammatory M1 to the anti-inflammatory M2-phenotype before resolving to the resting state.7 A shortcoming of our study is that neither PET imaging nor Iba-1 staining provided information about the phenotype of the activated microglia.
In contrast to its modulatory effect on microglia activation, exercise was not able to suppress the activation of astrocytes in the striatum 10 days after surgery in our study. Others, however, did observe a modulatory effect of exercise on 6-OHDA-induced astrocyte activation, which seemed to be associated with improved motor function.44 In that study, a higher dose of 6-OHDA (10 vs. 3 µg) was injected into the medial forebrain bundle, resulting in persistent astrocyte activation in sedentary animals up to day 32. Moreover, a more intense running protocol was used, which may have caused a greater effect on astrocytes. Despite contradictory evidence in the literature, our data suggest that exercise mainly has a modulatory effect on microglia rather than on astrocytes. In our study, astrocyte activation was only observed in dorsolateral striatum close to the injection site, both in control and PD animals. These data corroborate a previous study that observed an astrocyte reaction close to the needle track in rats injected with vehicle solution.45 These activated astrocytes might be mainly involved in restoration of damage to affected tissue by inducing glial scar formation.46
Our data showed that unilateral 6-OHDA injection had a detrimental effect on the dopaminergic system in sedentary animals, as demonstrated by both [18F]FDOPA PET and TH staining. However, dopaminergic damage in striatum appears to induce a compensatory response, since the reduction in TH staining was larger at day 10 after 6-OHDA injection than at day 30. In SNc, such a spontaneous recovery was not observed. This is in line with studies that revealed that surviving dopaminergic neurons increase the dopamine production, trying to compensate neuronal loss. Exercise can intensify this compensatory mechanisms.10
Exercise resulted in a partial reduction of dopaminergic damage in the striatum at day 10 and complete recovery at day 30. Exercise has been linked to protection of the dopaminergic system by increasing the release of neurotrophic factors that can have anti-inflammatory properties.5,7,8,10 The exercise-induced reduction in dopaminergic damage observed in our study seems, therefore, to be connected to the modulatory effect of exercise on glial activation in striatum, which could in turn have resulted in prevention of inflammation-induced neuronal damage. In SNc, however, exercise did not have any effect on TH staining at day 10 at all, but resulted in partial recovery of the dopaminergic damage at day 30. This indicates that there was a decrease in the TH expression at day 10, but neurons were not dead yet.
[18F]FDOPA PET results were highly correlated with postmortem TH staining and reduced striatal tracer uptake was associated with motor symptoms. Kyono et al.12 showed that tracer uptake correlated negatively with the severity of dopaminergic dysfunction. In another study, poor motor performance was directly linked to a decline in striatal [18F]FDOPA uptake.47 Prolonged reaction and movement time were related to lower [18F]FDOPA uptake in the caudate nucleus, and abnormalities in hand fine force control were related to striatal [18F]FDOPA uptake. These findings provide evidence that regional loss of nigrostriatal inputs to frontostriatal networks affects specific aspects of motor function.
A relatively mild model of PD was used in our study to enable detection of the beneficial effects of exercise. The mild severity of the model was confirmed by the observation that intra-striatal injection of the neurotoxin 6-OHDA only had an effect on body weight in the first week after surgery, but did not cause any impairment of motor activity in the open field test or cylinder test at day 28/29. Previous studies showed deficits in motor activity when 80% of the dopaminergic cells had been killed, while 55% of neuronal death did not change locomotion.48,49 Apparently, in our study, dopaminergic loss at day 28/29 was insufficient to evoke an effect on motor activity. On the other hand, at day 9 after 6-OHDA injection, the cylinder test detected significant asymmetry in forelimb use in sedentary rats. This apparent discrepancy could be ascribed to more severe loss of dopamine synthesis at this time point, as TH staining in striatum was reduced by approximately 50% at day 10, whereas only a 20% reduction was found at day 30. Another possible explanation for improved asymmetry forelimb use for SED + PD animals at day 29 may be a compensatory mechanism of the contralateral hemisphere, since the lesion of our model is unilateral.50 If a compensatory mechanism was present, however, it did not result in increased [18F]FDOPA uptake in the control hemisphere. In contrast, previous studies did reveal compensatory changes in the control hemisphere. This apparent discrepancy can be explained by the mild PD model used in our study, with dopaminergic loss being only 30–50%. Compensatory mechanisms in the contralateral hemisphere were described in unilateral PD models with dopaminergic loss of more than 60%.51–55
Interestingly, intermittently forced exercise reduced the degree of dopaminergic loss (−30%) at day 9 after 6-OHDA injection, resulting in normalization of forelimb use in the cylinder test. Apparently, there is a threshold for the amount of dopaminergic damage that is required to induce motor symptoms. Improvement in motor function as a result of exercise has been associated with increased plasticity and dendritic spines,56 which could have been induced by the release of neurotrophic factors.5,7,8,10
Non-motor symptoms, including memory impairment, usually already appear in early stages of PD and are increasingly recognized as a major challenge in the treatment. Our study showed that injection of 6-OHDA affects both short-term and long-term memory in sedentary animals. The role of the dopaminergic system in the NOR test has been demonstrated in pharmacological studies, in which D1 and D2/D3 antagonists were applied.57 6-OHDA-induced dopaminergic loss in caudate and substantia nigra leads to dysfunction of the prefrontal cortex, an area involved short-term memory.58 Previous studies have also linked microglia activation to non-motor symptoms in neurodegenerative diseases.59 We observed activation of microglia, not only in the dopaminergic system but also in the hippocampus, a brain region associated with memory function. Memory deficits observed in our study may therefore also be related to activation of microglia in hippocampus at day 10. Exercise was able to suppress microglia activation in hippocampus, which could also have been responsible for the prevention of the deleterious effects of 6-OHDA on memory. Moreover, exercise may have increased neuroplasticity of corticostriatal circuits, resulting in modulation of dopamine and glutamate neurotransmission, synaptogenesis, and cerebral blood flow.60 Thus, exercise may be able to improve cognitive function in PD by either preventing dopaminergic loss or suppressing glial activation.
Conclusions
The present study corroborates previous findings suggesting intermittent exercise could have the potential to become an (add-on) non-pharmacological intervention for PD. Exercise has a modulating effect on activation of microglia after exposure to 6-hydroxydopamine. Exercise-induced attenuation of microglia seems to have a beneficial effect on preservation and/or recovery of the dopaminergic system, cognition and motor function. The present study also demonstrated that PET enables noninvasive, longitudinal monitoring of the modulating effects of exercise on glial activation and degeneration of presynaptic dopaminergic neurons. The modulating effect of exercise, the 6-OHDA-induced glial activation measured by [11C]PBR28 PET was confirmed by Iba-1 staining, whereas [18F]FDOPA PET correlated well with TH staining and behavioral symptoms. These results warrant elaborate longitudinal studies assessing the beneficial effects of exercise on PD progression using PET.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was financially supported by travel grant from FAPESP - Brazil (Grant: 2014/23509-9). The authors thank Bram Maas, Rolf Zijlma, Chantal Kwizera, Janet Hessels-Scheper, Petra Maarsingh, Ate Boerema and Jurgen Sijbesma for their technical assistance.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
CCR, JD, DPF, LRGB and EFJV designed the experiments. CCR collected the data and wrote the paper. CCR, PKF and DVG analyzed the data. All the authors discussed data, edited, commented on the manuscript, and approved the final version of the manuscript.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Benetti F, Gustincich S, Legname G. Gene expression profiling and therapeutic interventions in neurodegenerative diseases: acomprehensive study on potentiality and limits. Expert Opin Drug Discov 2012; 7: 245–259. [DOI] [PubMed] [Google Scholar]
- 2.Dodel RC, Eggert KM, Singer MS, et al. Costs of drug treatment in Parkinson’s disease. Mov Disord 1998; 13: 249–254. [DOI] [PubMed] [Google Scholar]
- 3.Siderowf AD, Holloway RG, Stern MB. Cost-effectiveness analysis in Parkinson’s disease: determining the value of interventions. Mov Disord 2000; 15: 439–445. [DOI] [PubMed] [Google Scholar]
- 4.Baek SS. Role of exercise on the brain. J Exerc Rehabil 2016; 12: 380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.da Silva PG, Domingues DD, de Carvalho LA, et al. Neurotrophic factors in Parkinson’s disease are regulated by exercise: evidence-based practice. J Neurol Sci 2016; 363: 5–15. [DOI] [PubMed] [Google Scholar]
- 6.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 2002; 25: 295–301. [DOI] [PubMed] [Google Scholar]
- 7.Di Benedetto S, Müller L, Wenger E, et al. Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci Biobehav Rev 2017; 75: 114–128. [DOI] [PubMed] [Google Scholar]
- 8.Real CC, Ferreira AF, Chaves-Kirsten GP, et al. BDNF receptor blockade hinders the beneficial effects of exercise in a rat model of Parkinson’s disease. Neuroscience 2013; 237: 118–129. [DOI] [PubMed] [Google Scholar]
- 9.Cobianchi S, Arbat-Plana A, López-Álvarez VM, et al. Neuroprotective effects of exercise treatments after injury: the dual role of neurotrophic factors. Curr Neuropharmacol 2017; 15: 495–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zigmond MJ, Smeyne RJ. Exercise: is it a neuroprotective and if so, how does it work? Parkinsonism Relat Disord 2014; January(20 Suppl 1): S123–S127. [DOI] [PubMed] [Google Scholar]
- 11.Lau YS, Patki G, Das-Panja K, et al. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson’s disease with moderate neurodegeneration. Eur J Neurosci 2011; 33: 1264–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyono K, Takashima T, Katayama Y, et al. Use of [18F]FDOPA-PET for in vivo evaluation of dopaminergic dysfunction in unilaterally 6-OHDA-lesioned rats. EJNMMI Res 2011; 1: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eshuis SA, Jager PL, Maguire RP, et al. Direct comparison of FP-CIT SPECT and F-DOPA PET in patients with Parkinson’s disease and healthy controls. Eur J Nucl Med Mol Imaging 2009; 36: 454–462. [DOI] [PubMed] [Google Scholar]
- 14.Walker MD, Dinelle K, Kornelsen R, et al. Measuring dopaminergic function in the 6-OHDA-lesioned rat: a comparison of PET and microdialysis. EJNMMI Res 2013. a; 3: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker MD, Dinelle K, Kornelsen R, et al. In-vivo measurement of LDOPA uptake, dopamine reserve and turnover in the rat brain using [18F]FDOPA PET. J Cereb Blood Flow Metab 2013. b; 33: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerhard A, Pavese N, Hotton G, et al. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis 2006; 21: 404–412. [DOI] [PubMed] [Google Scholar]
- 17.Albrecht DS, Granziera C, Hooker JM, et al. In vivo imaging of human neuroinflammation. ACS Chem Neurosci 2016; 7: 470–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupont AC, Largeau B, Santiago Ribeiro MJ, et al. Translocator protein-18 kDa (TSPO) positron emission tomography (PET) imaging and its clinical impact in neurodegenerative diseases. Int J Mol Sci 2017; 18 pii: E785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papadopoulos V, Baraldi M, Guilarte TR, et al. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 2006; 27: 402–409. [DOI] [PubMed] [Google Scholar]
- 20.Tronel C, Largeau B, Santiago Ribeiro MJ, et al. Molecular targets for PET imaging of activated microglia: the current situation and future expectations. Int J Mol Sci 2017; 18 pii: E802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parente A, Kopschina Feltes P, Vállez García D, et al. Pharmacokinetic analysis of 11C-PBR28 in the rat model of herpes encephalitis (HSE): comparison with (R)-11C-PK11195. J Nucl Med 2016; 57: 785–791. [DOI] [PubMed] [Google Scholar]
- 22.Schweitzer PJ, Fallon BA, Mann JJ, et al. PET tracers for the peripheral benzodiazepine receptor and uses thereof. Drug Discov Today 2010; 15: 933–942. [DOI] [PubMed] [Google Scholar]
- 23.Jucaite A, Svenningsson P, Rinne JO, et al. Effect of the myeloperoxidase inhibitor AZD3241 on microglia: a PET study in Parkinson’s disease. Brain 2015; 138(Pt 9): 2687–2700. [DOI] [PubMed] [Google Scholar]
- 24.Real CC, Garcia PC, Britto LRG. Treadmill exercise prevents increase of neuroinflammation markers involved in the dopaminergic damage of the 6-OHDA Parkinson’s disease model. J Mol Neurosci 2017; 63: 36–49. [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 7th ed San Diego: Academic Press, 2013. [Google Scholar]
- 26.Garcia PC, Real CC, Britto LR. The impact of short and long-term exercise on the expression of arc and AMPARs during evolution of the 6-hydroxy-dopamine animal model of Parkinson’s disease. J Mol Neurosci 2017; 61: 542–552. [DOI] [PubMed] [Google Scholar]
- 27.Goes AT, Souza LC, Filho CB. Neuroprotective effects of swimming training in a mouse model of Parkinson’s disease induced by 6-hydroxydopamine. Neuroscience 2014; 256: 61–71. [DOI] [PubMed] [Google Scholar]
- 28.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 2003; 463: 3–33. [DOI] [PubMed] [Google Scholar]
- 29.Barbiero JK, Santiago RM, Persike DS, et al. Neuroprotective effects of peroxisome proliferator-activated receptor alpha and gamma agonists in model of parkinsonism induced by intranigral 1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine. Behav Brain Res 2014; 274: 390–399. [DOI] [PubMed] [Google Scholar]
- 30.Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 2012; 13: 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res 1988; 31: 47–59. [DOI] [PubMed] [Google Scholar]
- 32.Santos JR, Cunha JA, Dierschnabel AL, et al. Cognitive, motor and tyrosine hydroxylase temporal impairment in a model of parkinsonism induced by reserpine. Behav Brain Res 2013; 253: 68–77. [DOI] [PubMed] [Google Scholar]
- 33.Carlini VP, Martini AC, Schiöth HB, et al. Decreased memory for novel object recognition in chronically food-restricted mice is reversed by acute ghrelin administration. Neuroscience 2008; 153: 929–934. [DOI] [PubMed] [Google Scholar]
- 34.Brooks SP, Dunnett SB. Tests to assess motor phenotype in mice: a user’s guide. Nat Rev Neurosci 2009; 10: 519–529. [DOI] [PubMed] [Google Scholar]
- 35.Boix J, Padel T, Paul G. A partial lesion model of Parkinson’s disease in mice – characterization of a 6-OHDA-induced medial forebrain bundle lesion. Behav Brain Res 2015; 284: 196–206. [DOI] [PubMed] [Google Scholar]
- 36.Schallert T, Fleming SM, Leasure JL, et al. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 2000; 39: 777–787. [DOI] [PubMed] [Google Scholar]
- 37.Vercammen L, Van der Perren A, Vaudano E, et al. Parkin protects against neurotoxicity in the 6-hydroxydopamine rat model for Parkinson’s disease. Mol Ther 2006; 14: 716–723. [DOI] [PubMed] [Google Scholar]
- 38.Vállez García D, Casteels C, Schwarz AJ, et al. A standardized method for the construction of tracer specific PET and SPECT rat brain templates: validation and implementation of a toolbox. PLoS One 2015; 10: e0122363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumakura Y, Cumming P. PET studies of cerebral levodopa metabolism: a review of clinical findings and modeling approaches. Neuroscientist 2009; 15: 635–650. [DOI] [PubMed] [Google Scholar]
- 40.Kordys E, Apetz N2, Schneider K, et al. Motor impairment and compensation in a hemiparkinsonian rat model: correlation between dopamine depletion severity, cerebral metabolism and gait patterns. EJNMMI Res 2017; 7: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardin JW, Hilbe JM. Generalized estimating equations, Boca Raton, FL: Chapman & Hall/CRC Press, 2012. [Google Scholar]
- 42.Marinova-Mutafchieva L, Sadeghian M, Broom L, et al. Relationship between microglial activation and dopaminergic neuronal loss in the substantia nigra: a time course study in a 6-hydroxydopamine model of Parkinson’s disease. J Neurochem 2009; 110: 966–975. [DOI] [PubMed] [Google Scholar]
- 43.Perry VH. Innate inflammation in Parkinson’s disease. Cold Spring Harb Perspect Med 2012; 2: a009373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dutra MF, Jaeger M, Ilha J, et al. Exercise improves motor deficits and alters striatal GFAP expression in a 6-OHDA-induced rat model of Parkinson’s disease. Neurol Sci 2012; 33: 1137–1144. [DOI] [PubMed] [Google Scholar]
- 45.Gomide VC, Silveira GA, Chadi G. Transient and widespread astroglial activation in the brain after a striatal 6-OHDA-induced partial lesion of the nigrostriatal system. Int J Neurosci 2005; 115: 99–117. [DOI] [PubMed] [Google Scholar]
- 46.Becerra-Calixto A, Cardona-Gómez GP. The role of astrocytes in neuroprotection after brain stroke: potential in cell therapy. Front Mol Neurosci 2017; 10: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gallagher CL, Johnson SC, Bendlin BB, et al. A longitudinal study of motor performance and striatal [18F]fluorodopa uptake in Parkinson’s disease. Brain Imaging Behav 2011; 5: 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, Zhang Y, Li L, et al. Neuroprotective effects of geniposide in the MPTP mouse model of Parkinson’s disease. Eur J Pharmacol 2015; 768: 21–27. [DOI] [PubMed] [Google Scholar]
- 49.Fornaguera J, Schwarting RK. Early behavioral changes after nigro-striatal system damage can serve as predictors of striatal dopamine depletion. Prog Neuropsychopharmacol Biol Psychiatry 1999; 23: 1353–1368. [DOI] [PubMed] [Google Scholar]
- 50.Seeger-Armbruster S, von Ameln-Mayerhofer A. Short- and long-term unilateral 6-hydroxydopamine lesions in rats show different changes in characteristics of spontaneous firing of substantia nigra pars reticulata neurons. Exp Brain Res 2013; 224: 15–24. [DOI] [PubMed] [Google Scholar]
- 51.Zhang WQ, Tilson HA, Nanry KP, et al. Increased dopamine release from striata of rats after unilateral nigrostriatal bundle damage. Brain Res 1988; 461: 335–342. [DOI] [PubMed] [Google Scholar]
- 52.Robinson TE, Castañeda E, Whishaw IQ. Compensatory changes in striatal dopamine neurons following recovery from injury induced by 6-OHDA or methamphetamine: a review of evidence from microdialysis studies. Can J Psychol 1990; 44: 253–275. [DOI] [PubMed] [Google Scholar]
- 53.Zigmond MJ, Abercrombie ED, Berger TW, et al. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci 1990; 13: 290–296. [DOI] [PubMed] [Google Scholar]
- 54.Song DD, Haber SN. Striatal responses to partial dopaminergic lesion: evidence for compensatory sprouting. J Neurosci 2000; 20: 5102–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deumens R, Blokland A, Prickaerts J. Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol 2002; 175: 303–317. [DOI] [PubMed] [Google Scholar]
- 56.Shin MS, Jeong HY, An DI, et al. Treadmill exercise facilitates synaptic plasticity on dopaminergic neurons and fibers in the mouse model with Parkinson’s disease. Neurosci Lett 2016; 621: 28–33. [DOI] [PubMed] [Google Scholar]
- 57.Bevins RA, Besheer J, Palmatier MI, et al. Novel-object place conditioning: behavioral and dopaminergic processes in expression of novelty reward. Behav Brain Res 2002; 129: 41–50. [DOI] [PubMed] [Google Scholar]
- 58.Jokinen P, Karrasch M, Brück A, et al. Cognitive slowing in Parkinson ‘ s disease is related to frontostriatal dopaminergic dysfunction. J Neurol Sci 2013; 329: 23–28. [DOI] [PubMed] [Google Scholar]
- 59.Doty KR, Town T. The role of the immune system in neurodegenerative disorders : adaptive or maladaptive? Brain Res 2015; 1617: 155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petzinger GM, Holschneider DP, Fisher BE, et al. The effects of exercise on dopamine neurotransmission in Parkinson’s disease: targeting neuroplasticity to modulate basal ganglia circuitry. Brain Plast 2015; 1: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.