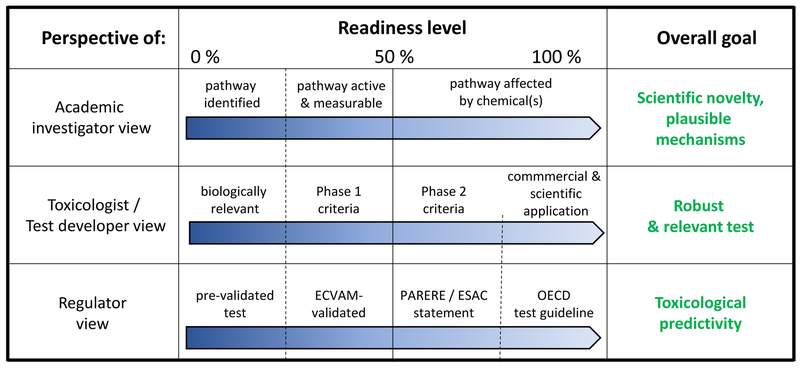
Figure 2: Different perspectives of DNT alternative methods readiness evaluation.
In the discussion on “test readiness” it is important to note that different fields and stakeholders have their own perspective. Three of these perspectives are outlined. For each of them, examples for increasing grades of readiness and final goals are given. These perspectives are interdependent to some degree: (i) a test that is 100% ready for an academic investigator in basic science can form the starting point for a toxicological test developer; (ii) a test that is considered ready by the test developer may be at the start of regulatory readiness, e.g. with respect to formal validation; (iii) and a test that is at the highest regulatory readiness level (OECD TG) may provide a starting point for academic researchers who want to unravel key mechanisms and pathways that are essential and that biologically explain the test read outs.