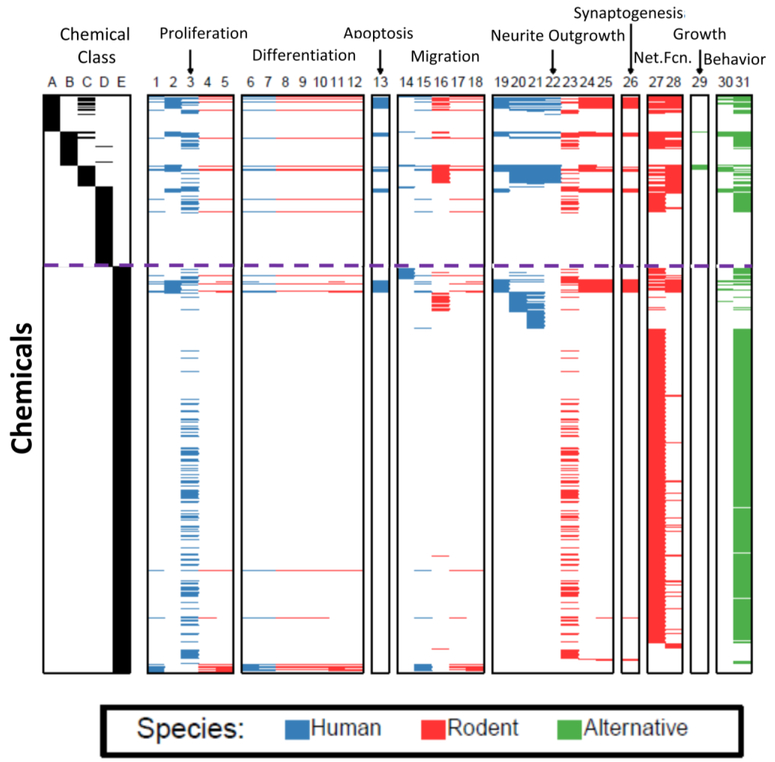
Figure 5. The current chemical landscape of in vitro DNT testing.
The heatmap plots chemicals as rows and test status as columns. The first 5 columns provide evidence of the class of chemicals relative to evidence of DNT or priority for testing (see main text chapter 5.1). The other columns list assays grouped by neurodevelopmental processes. A brief description of each column is provided below, along with a reference or references, if available. Compounds from columns A-E that have been tested in different assays (columns 1–31), are indicated by a blue (human), red (rodent), or green (alternative species) horizontal line. It should be noted that the information on what compounds have been tested was provided by the laboratories engaged in testing, and that not all of the data for each compound/assay pair have been published. Chemical class columns: A Compounds with evidence of developmental neurotoxicity from multiple laboratories (Mundy et al., 2015); B Compounds with evidence of developmental neurotoxicity from only 1 laboratory (Mundy et al., 2015); C Compounds in the 87 chemical library supplied by the National Toxicology Program; D Compounds subjected to the literature search in Mundy et al., 2015 that did not have evidence of developmental neurotoxicity; E Other compounds. This consists primarily of ToxCast compounds, but also assay positive controls and other miscellaneous compounds. Assay columns: 1 Proliferation in human neurospheres (Baumann et al., 2016); 2 Proliferation in hNP1 neuroprogenitor cells (Mundy et al., 2010); 3 Proliferation in ReNcellCX human neuroprogenitors (Breier et al., 2008; Radio et al., 2015); 4 Proliferation in mouse neurospheres (Fritsche et al., unpublished data); 5 Proliferation in rat neurospheres (Baumann et al., 2016); 6 Neuronal differentiation in human neurospheres (Baumann et al., 2016); 7 Oligodendrocyte differentiation in human neurospheres (Fritsche et al., unpublished data); 8 Differentiation in mouse neurospheres (Fritsche et al., unpublished data); 9 Neuronal differentiation in mouse neurosphere (Fritsche et al., unpublished data)10 Oligodendrocyte differentiation in mouse neurospheres (Fritsche et al., unpublished data); 11 Neuronal differentiation in rat neurospheres (Baumann et al., 2016); 12 Oligodendrocyte differentiation in rat neurospheres (Fritsche et al., unpublished data); 13 Apoptosis in human NP1 neural precursors (Druwe et al., 2015); 14 Migration of human neuroprogenitor cells; 15 Migration in human neurospheres (Baumann et al., 2016); 16 Migration in human neural crest cells (Nyeffler et al., 2017a, Nyeffler et al., 2017b); 17 Migration in mouse neurospheres (Fritsche et al., unpublished data); 18 Migration in rat neurospheres (Baumann et al.,2016); 19 Neurite outgrowth in human hN2 neurons. (Harrill et al., 2010); 20 Neurite outgrowth in human peripheral neuroprecursors (Hoelting et al., 2016); 21 Neurite outgrowth in LUHMES neurons (Krug et al., 2013b); 22 Neurite outgrowth in human iPS-derived neurons. (Ryan et al., 2016); 23 Neurite outgrowth in PC12 cells (Radio et al., 2015); 24 Neurite outgrowth in rat cortical neurons (Harrill et al., 2011a); 25 Maturation of neurites in rat cortical neurons (Harrill et al., 2011b); 26 Synaptogenesis in primary cortical neurons (Harrill et al., 2011b); 27 Neuronal Network Function- Acute (Strickland et al., 2017, in press); 28 Neuronal Network Formation- Developmental (Brown et al.,2016); 29 Feeding, larval development and reproduction in C. elegans (Behl et al., 2016.); 30 Zebrafish behavior- (Cowden et al., 2012: Padilla et al., 2011); 31 Zebrafish behavior 24 hr post-fertilization (Reif et al., 2016).