Fig. 2 |. Vaccination induces circulating neoantigen-specific T cell responses in patients who did not receive dexamethasone during vaccine priming.
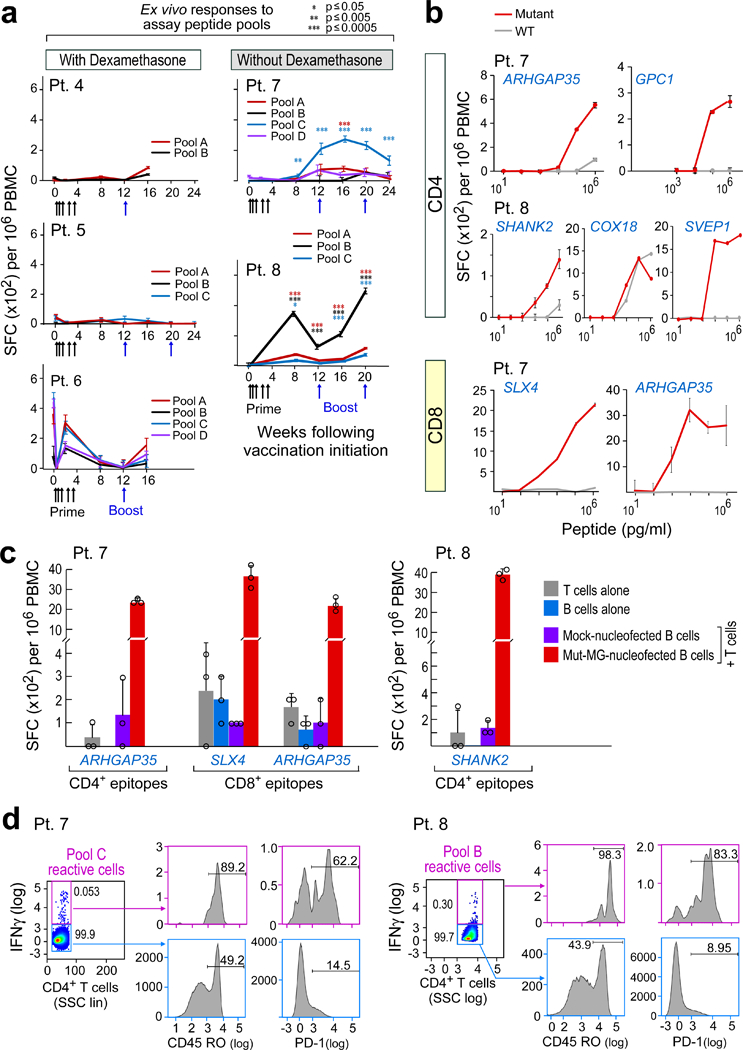
a, Ex vivo IFNγ ELISPOT responses for PBMCs that were stimulated with neoantigen-peptide pools. Data are background-subtracted; n = 3 biologically independent samples, data are mean ± s.e.m.; Wilcoxon signed-rank test, two-sided without adjustment for multiple comparisons, see Methods for statistical analysis; SFC, spot- forming cells. b, IFNγ secretion by neoantigen-reactive T cell lines from patients 7 and 8 shows discrimination between mutated and wild-type peptides, focusing on neoantigens generating CD4+ and CD8+ T cell responses. n = 3 biological independent samples, data are mean ± s.d.; SHANK2 responses were detected ex vivo. c, Dominant responses of CD4+ and CD8+ neoantigen-specific T cell lines from patients 7 and 8 are directed against endogenously processed and presented peptides, as shown by reactivity of these cells against autologous B cells that were nucleofected with minigenes (MG) that encoded these neoantigens. n = 3 biologically independent samples, data are mean ± s.d. d, Ex vivo intracellular cytokine staining of PBMCs from patients 7 and 8 post-vaccination after neoepitope stimulation. PBMCs were pre-gated on CD3+ and CD4+ T cells. Cytokine-producing neoantigen-reactive cells express CD45RO and PD-1, demonstrating an antigen-experienced memory T cell phenotype. SSC, side scatter; All T cell lines originated from week 8 or 16 PBMCs; ELISPOT experiments were performed in triplicate wells per time point.
