Fig. 4 |. TCRαβ clonotypes are shared between tumour-associated T cells and neoantigen-reactive T cells in peripheral blood, with select clones demonstrated to be specific for neoantigens targeted by the vaccine.
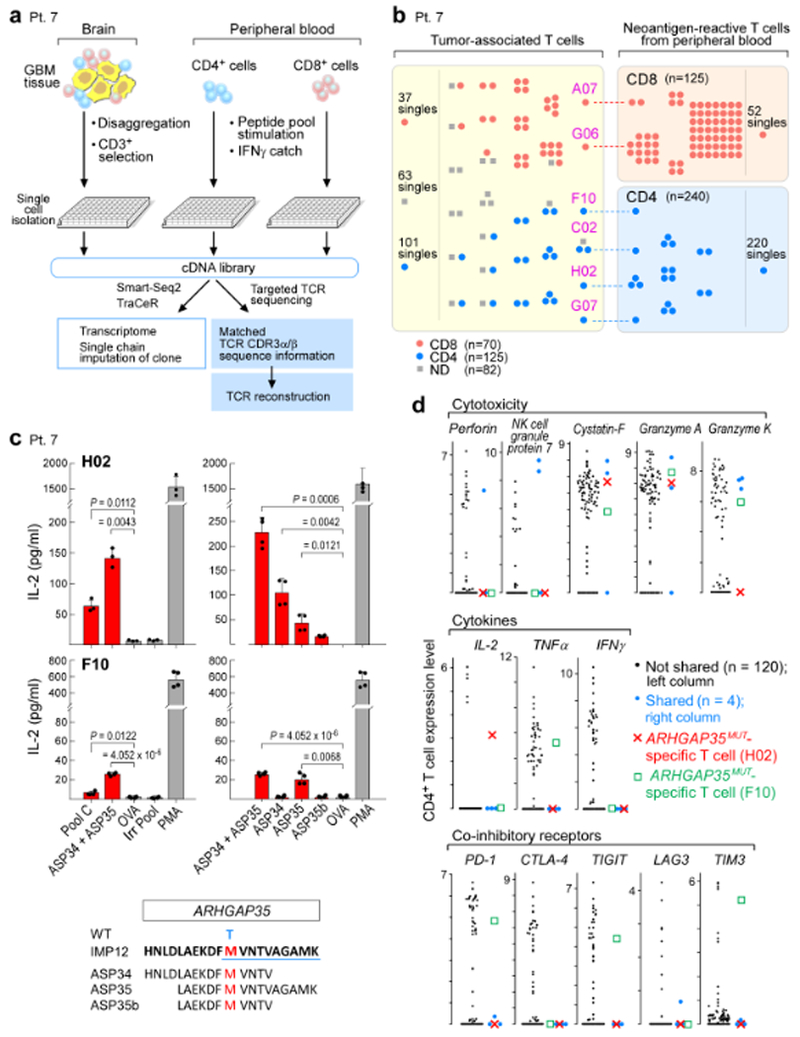
a, Schema of single-cell transcriptome and TCR analysis of T cells isolated from the relapse tumour specimen, and from PBMCs stimulated in vitro with immunizing neoantigens from patient 7. b, Select TCRαβ clones are shared between tumour-associated T cells and neoantigen-reactive T cells in the periphery. Each dot represents a single T cell, and cells with identical TCRs are clustered together. c, CD4+ TCRs H02 and F10, expressed in JurkatΔαβ cells, are specific for ARHGAP35MUT, a neoantigen targeted by vaccination for patient 7. H02 TCR responds predominantly to ASP34, whereas F10 responds to ASP35. Two-sample two-sided t-tests with Welch correction used for comparison to negative control ovalbumin peptide (OVA); Irr Pool, irrelevant peptide pool; PMA, phorbol 12-myristate 13-acetate; WT, wild- type amino acid. Top left n = 3 biologically independent samples, all others n = 2 biologically independent samples, each with two technical replicates. Representative data from one of three independent experiments are shown; data are mean ± s.d. d, Distribution of expression levels (log2(transcripts per million/10 + 1)) for select genes in CD4+ infiltrating T cells, highlighting the ARHGAP35MUT-specific T cells, H02 and F10; blue dots, other cells with TCR clones shared between tumour-associated and neoantigen-reactive T cells in peripheral blood.
