Figure 3.
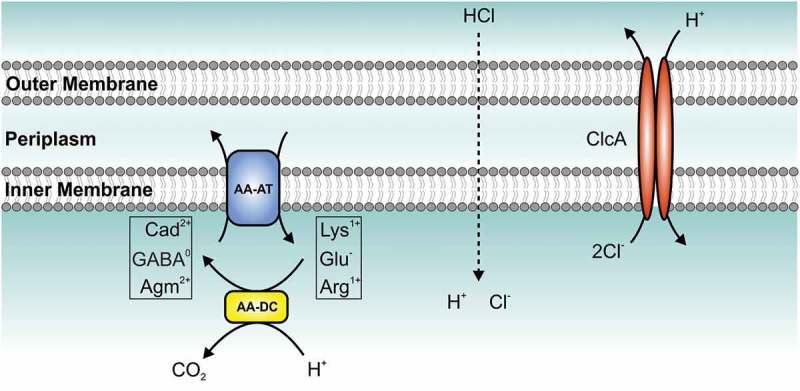
Model of the amino acid-dependent decarboxylation system and ClcA (H+/Cl− transporter) within the bacterial acid tolerance response. Upon exposure to hydrochloric acid (HCl), bacteria activate the amino acid-dependent decarboxylation system: amino acid decarboxylase (AA-DC, yellow) convert amino acids [lysine (Lys1+), glutamate (Glu−) or arginine (Arg1+)] to their decarboxylated versions [cadaverine (Cad2+), γ-aminobutyric acid (GABA°), or agmatine (Agm2+)] thereby consuming a proton (H+) and producing carbon dioxide (CO2). A coupled, electrogenic amino acid-amine antiporter (AA-AT, blue) located in the membrane removes the product and provides new substrate via exchange of Cad2+ and Lys1+, GABA° and Glu− or Agm2+ and Arg1+, respectively. The H+/Cl− transporter (ClcA, red) exports two chloride-ions (Cl−) in exchange of one proton (H+) to detoxify the chloride and acts as electrical shunt to prevent excessive inner-membrane hyperpolarization, which would paralyze the system. However, under alkaline conditions an active H+/Cl− transporter would exploit the proton motive force thereby depleting the energy of the bacterial cell. Thus, expression of clcA is high in the stomach, but V. cholerae represses clcA during colonization of the intestine representing an alkaline environment.
