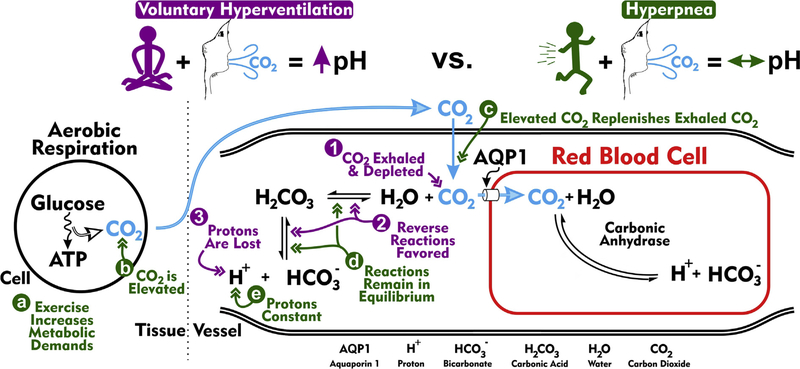
Fig. 2.
Acid-base blood physiology during rapid breathing. The schematic depicts biochemical reactions largely responsible for establishing blood pH. On the left is shown a cell situated in the tissue undergoing aerobic respiration, the process of converting glucose into ATP. Carbon dioxide (CO2) is produced during this conversion process. CO2 then diffuses into capillary plasma, after which the bulk is primarily transported into erythrocytes (red blood cells) via aquaporin 1 (AQP1) channels (Endeward, 2006). Within the red blood cell, CO2 associates with H2O (water) to produce H+ (proton) and (bicarbonate), a reaction catalyzed by carbonic anhydrase. Carbonic anhydrase is found in abundance in red blood cells, but not in the plasma. The dissociated proton acidifies the red blood cell and, in doing so, promotes the dissociation of hemoglobin and oxygen. A small portion of CO2 entering the capillary remains in the plasma. As in the red blood cell, plasma CO2 is also converted into H+ and , but this reaction is primarily not catalyzed by carbonic anhydrase [i.e. an uncatalyzed reaction (however, some carbonic anhydrase isoforms are localized to the extracellular surface of many cells (Boron, 2010; Svichar and Chesler, 2003), including red blood cells, and contribute to the catalyzed production of H+ and from CO2 and H2O (Svichar and Chesler, 2003)]. The uncatalyzed reaction reversibly proceeds through H2CO3 (carbonic acid) and occurs at low basal rates. Purple (1–3). Acid-base physiology during voluntary hyperventilation. Voluntary hyperventilation is defined by excessive breathing (high rate and quantity) leading to the pronounced ventilation of CO2 and ensuing respiratory alkalosis, a phenomenon in which blood plasma becomes alkaline. (1) Excessive ventilation reduces plasma concentrations of CO2. (2) CO2 depletion promotes the association of plasma H+ and to replenish, via H2CO3, CO2. (3) Favoring the reverse reactions reduces the concentration of plasma H+, thereby making the blood more alkaline. Green (a–e). Acid-base physiology during exercise-related hyperventilation (i.e. hyperpnea). (a) Unlike during voluntary hyperventilation, hyperpnea occurs during times of heightened metabolic demand. (b) Aerobic respiration associated with high metabolic demands produces high levels of CO2. (c) Increased CO2 production and subsequent diffusion into capillaries balances the increased CO2 exhalation. (d) The equilibria underlying CO2-H2O biochemical reactions remain unperturbed. (e) As these biochemical reactions remain in equilibrium, plasma protons are not lost and plasma pH is stable. Importantly, absence seizures are triggered by voluntary hyperventilation, but not hyperpnea.