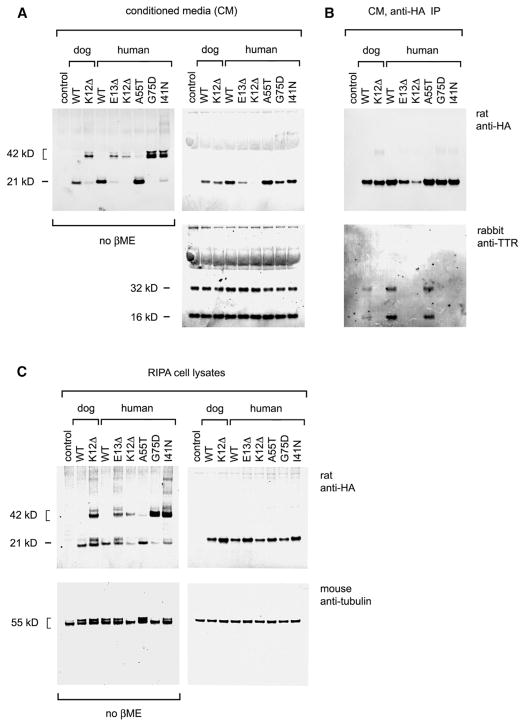
Figure 5. RBP Secretion and Transthyretin Binding In Vitro.
(A–C) LI-COR western analysis of (A) CM, (B) immunoprecipitates (IP) of CMs, and (C) radio-immunoprecipitation assay buffer (RIPA) lysates from transfected HeLa cells expressing dog (WT and K12del) or human (WT, K12del, E13del, A55T, G75D, and I47N) HARBPs, detected using anti-HA antibody. In (A) and (B), parallel gels were electrophoresed under reducing (+βME, right) or non-reducing conditions (no βME, left), with bovine TTR and human α-tubulin as CM and lysate loading controls, respectively.
(A) Recombinant HARBPs are secreted as dimers or higher multimers (K12del in both species; human E13del, G75D, and I41N) and monomers (WT in both species, human A55T). The K12del dimers secreted by HeLa cells in vitro appear similar to RBP dimers secreted by mutant dogs in vivo, and presumably they reflect the exposure of unpaired cysteines, which persist after intra-molecular oxidative folding. The A55T, G75D, and I41N proteins behave as previously reported (Chou et al., 2015).
(B) The RBP profiles of transfected cell lysates and CMs generally correspond; however, mutant monomers are notably more abundant (>50%) in cell lysates than in CMs. These abnormal RBPs are presumably retained in the ER and secreted following dimerization.
(C) Co-immunoprecipitation showing that only WT and stable A55T mutant HARBPs interact with bovine TTR present in the CMs.
See also Figure S3.