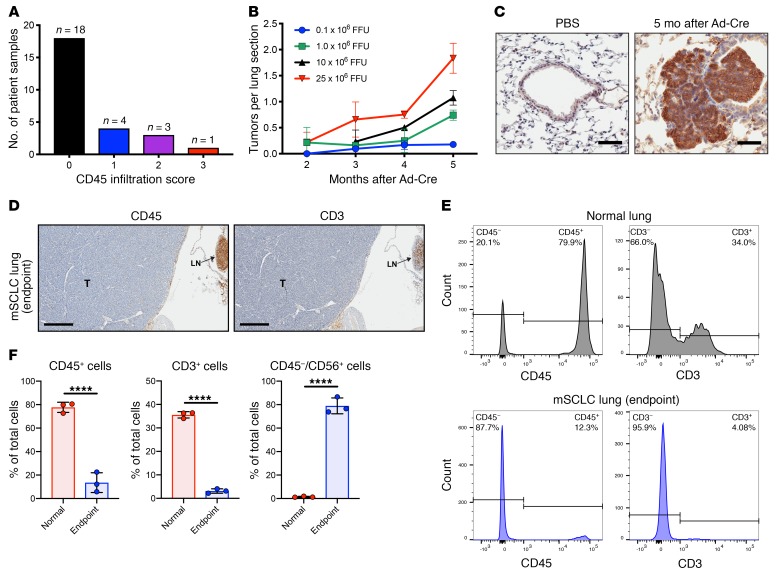
Figure 2. SCLC patient specimens and a genetically engineered mouse model for SCLC show scant immune cell infiltration.
(A) Data from 26 SCLC patient specimens showing CD45 infiltration score (0–3+) defined by CD45+ immunostaining. (B) Dose-dependent study of Ad-Cre delivered by intratracheal injection showing the number of tumor lesions observed at the indicated time points for each Ad-Cre dose. Results are representative of 3 animals per group, and data indicate mean + SD. (C) NCAM-1 (CD56) immunohistochemistry 5 months after intratracheal delivery of Ad-Cre compared with PBS-treated control. Scale bars, 50 μm. (D) IHC of FFPE sections from a p53–/–/Rb1–/–/p130–/– mouse at survival endpoint; CD45 IHC shows negligible infiltrating immune cells and CD3 IHC shows negligible infiltrating T lymphocytes. The advanced SCLC lesion (T) occupies majority of the section with lymph node (LN) upper right confirming CD45 and CD3 reactivity. Scale bars, 400 μm. (E) CD45 and CD3 populations from an enzymatically digested whole lung obtained from adult normal lung (no Ad-Cre tumor induction) (top) or p53–/–/Rb1–/–/p130–/– mouse at survival endpoint (bottom) that confirms IHC results showing few immune cells (CD45+) and T lymphocytes (CD3+) in endpoint SCLC lung. (F) Quantification from whole-lung tissue using control (no Ad-Cre induction; n = 3) and SCLC endpoint (n = 3) showing reduction in immune CD45+ or CD3+ cells and presence of CD45–/CD56+ SCLC tumor cells. Bar showing mean + SD with all data points. ****P < 0.0001 by unpaired Student’s t test.