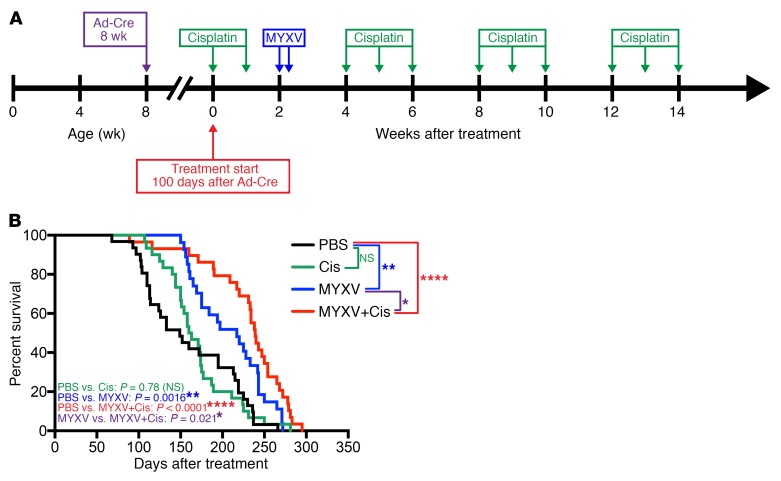
Figure 5. MYXV increases overall survival in conditional p53–/–/Rb1–/–/p130–/– SCLC GEMM.
Survival is enhanced when MYXV is combined with low-dose cisplatin. (A) Timeline for survival experiment indicating time of Ad-Cre tumor induction at 6–8 weeks of age, treatment start at 100 days after tumor induction, and the indicated time points for the therapeutic intervention. Mice receiving vMyx-M135KO-GFP were given 2 doses (5 × 107 FFU in 60 μl PBS) 48 hours apart, and cisplatin was administered by i.p. injection (2.5 mg/kg in 100 μl PBS) at the indicated time points. (B) Kaplan-Meier survival analysis of the experiment outlined in Figure 3A. Conditional p53–/–/Rb1–/–/p130–/– SCLC mice were randomized into 4 treatment groups. PBS control mice received intranasal instillation and i.p. injections of PBS. Mice treated with MYXV alone received i.p. injections of PBS at the same cisplatin treatment intervals, and the cisplatin-only group received intranasal instillation of PBS at the MYXV treatment interval. Survival data represent 31 mice in the PBS group, 30 mice in the cisplatin (Cis) group, 27 mice in the MYXV group, and 29 mice in the MYXV+Cis group. *P < 0.05, **P < 0.01, ****P < 0.0001, NS indicates P > 0.05, by log-rank (Mantel-Cox) test. Median survival for each treatment group was as follows: PBS: 149 days (95% CI, 113–213 days); Cis: 161.5 days (95% CI, 150–175 days); MYXV: 217 days (95% CI, 169–243 days); MYXV+Cis: 239 (95% CI, 220–254 days).