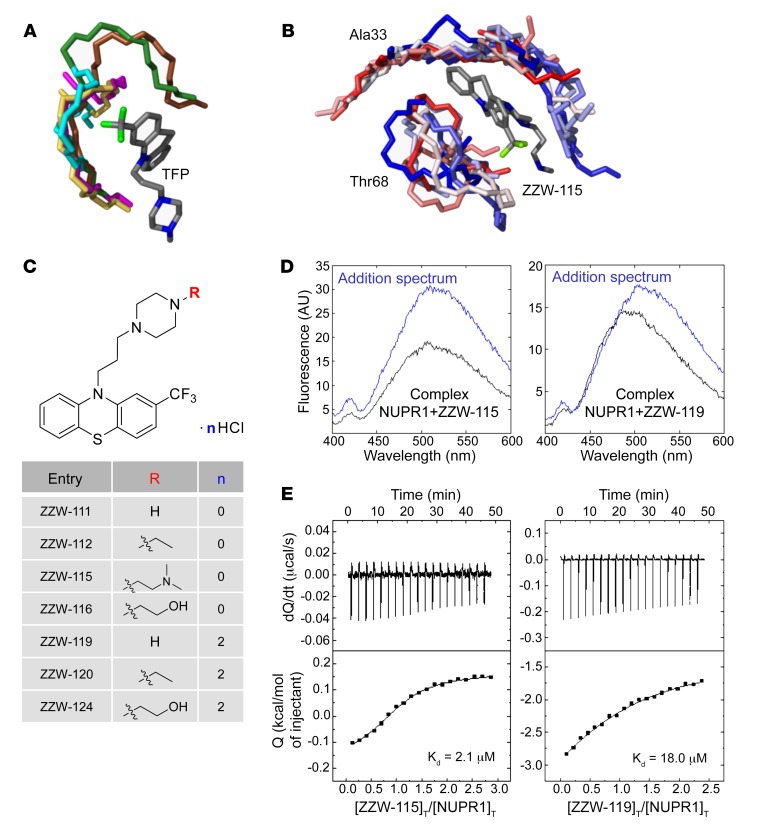
Figure 1. Synthesis of the compounds, and docking and biophysical characterization of NUPR1 to the TFP-derived compounds.
(A) Docking of TFP to short fragments of NUPR1 sequence (in different colors), mimicking potential binding locations. The phenothiazine and trifluoromethyl groups of TFP are critical for anchoring, whereas the rest of the structure contributes less. (B) Example of simulation of ZZW-115 (a single representative structure is shown in gray) in complex with sequence segments of NUPR1 (ensembles are in gradients of color, from red to blue); the labels indicate the central protein residue for each of the two 7-residue fragments. (C) Structure of the synthesized TFP-derived compounds. (D) Fluorescence ANS spectra in the presence or absence of ZZW-115 (left) and ZZW-119 (right) for the complex between both molecules (black) and for that obtained from the addition of the spectra of both isolated molecules: NUPR1 and the TFP-derived compound (blue). Experiments were performed in 20 mM sodium phosphate pH 7.0 at 25°C. (E) Calorimetric titrations corresponding to the interaction of ZZW-115 (left) and ZZW-119 (right) with NUPR1. Thermograms (thermal power as a function of time) are shown on the top, and binding isotherms (ligand-normalized heat effects as a function of molar ratio) are shown on the bottom. Experiments were performed at 25°C in sodium phosphate 20 mM, pH 7, 2% DMSO, with 20 μM NUPR1 in the calorimetric cell and 200 μM compound in the titrating syringe, using an Auto-iTC200 instrument (MicroCal-Malvern Panalytical).