Abstract
Normal proliferation and differentiation of uterine epithelial cells are critical for uterine development and function. Enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2), a core component of polycomb repressive complexes 2, possesses histone methyltransferase activity that catalyzes the trimethylation of lysine 27 of histone H3. EZH2 has been involved in epithelial-mesenchymal transition, a key event in development and carcinogenesis. However, its role in uterine epithelial cell function remains unknown. To determine the role of uterine EZH2, Ezh2 was conditionally deleted using progesterone receptor Cre recombinase, which is expressed in both epithelial and mesenchymal compartments of the uterus. Loss of EZH2 promoted stratification of uterine epithelium, an uncommon and detrimental event in the uterus. The abnormal epithelium expressed basal cell markers, including tumor protein 63, cytokeratin 5 (KRT5), KRT6A, and KRT14. These results suggest that EZH2 serves as a guardian of uterine epithelial integrity, partially via inhibiting the differentiation of basal-like cells and preventing epithelial stratification. The observed epithelial abnormality was accompanied by fertility defects, altered uterine growth and function, and the development of endometrial hyperplasia. Thus, the Ezh2 conditional knockout mouse model may be useful to explore mechanisms that regulate endometrial homeostasis and uterine function.
The endometrium of the uterus comprises both luminal and glandular epithelia, the integrity of which is critical for uterine function. In mice, the proliferation of luminal epithelium is reduced before blastocyst implantation; and high proliferative activity of luminal epithelium, resulting from imbalanced hormonal signaling, prevents blastocyst implantation.1 Enhanced uterine epithelial proliferation has also been linked to endometrial hyperplasia, a premalignant condition of endometrial cancer.2 It was shown that uterine epithelial stratification, arising from ablation of fibroblast growth factor receptor 2 (FGFR2), causes pregnancy complications.3 The murine endometrium contains slightly coiled glands that are formed postnatally.4 Uterine glands play an indispensable role in mice during pregnancy via the production of secretory substances that regulate uterine receptivity and decidualization.5, 6 The requirement of uterine glands in pregnancy was also demonstrated by using the ovine uterine gland knockout model.7 Furthermore, the importance of uterine gland secretions in mouse pregnancy was shown by studies using uterine-specific knockout mice of forkhead box A2 (Foxa2), in which the production of leukemia inhibitory factor essential for implantation is impaired.8
Polycomb group proteins are conserved epigenetic regulators of gene silencing and cell fate determination and maintenance.9 Two major polycomb group complexes, polycomb repressive complex (PRC) 1 and PRC2, have been characterized in mammals. The core subunits of PRC2 consist of enhancer of zeste 2 PRC 1 or 2 subunits (EZH1 or EZH2, respectively), SUZ12 polycomb repressive complex 2 subunit, and embryonic ectoderm development.10 Among these proteins, EZH2 is a well-established histone methyltransferase, catalyzing the trimethylation of lysine 27 of histone H3 (H3K27)10 or functionally interacting with DNA methyltransferases.11 EZH2 regulates a wide spectrum of physiological and pathologic events, including, but not limited to, cell differentiation, germline development, X-chromosome inactivation, stem cell pluripotency, cell-cycle progression, DNA repair, and cancer development.12, 13, 14, 15
Evidence supports a link between altered expression of EZH2 and in vitro decidualization of human endometrial cells,16 environmental estrogen exposure,17 uterine fibroids,15 endometriosis,18 and endometrial cancer development.19 However, the role of EZH2 in normal uterine epithelial cells remains undefined. Our results reveal that loss of EZH2 produces basal-like epithelial cells or a cell population expressing basal markers in the uterus, signifying the importance of epigenetic regulators in the female reproductive tract.
Materials and Methods
Animals and Tissue Collection
The animal use protocol was approved by the Texas A&M University (College Station, TX) Institutional Animal Care and Use Committee. Animals were handled according to the animal care and use guidelines from the NIH's Guide for the Care and Use of Laboratory Animals20 and the Texas A&M University Institutional Animal Care and Use Committee. Mice were housed under a 12-hour light/12-hour dark cycle in the Laboratory Animal Resources and Research facility. Animal care was provided by experienced technicians from the Comparative Medicine Program. Mice were on a C57BL/6; 129SvEv background. Generation of progesterone receptor (Pgr)–Cre mice21 and Ezh2flox/flox mice22 was described previously. The Ezh2flox/flox mice were purchased from The Jackson Laboratory (Bar Harbor, ME; stock number 022616). Vaginal smears were analyzed to confirm the cycling, and all mice used in this experiment had intact ovaries. Uterine epithelia were collected by a mild trypsin digestion,23 followed by mechanical separation using fine forceps under a stereo dissection microscope (Olympus, Waltham, MA). Uterine samples used for histologic studies were fixed with 10% (v/v) neutral-buffered formalin (Millipore Sigma, St. Louis, MO). Samples were embedded and processed using the on-campus Core Histology Laboratory of the Veterinary Integrative Biosciences Department. Hematoxylin and eosin staining was used to determine general histopathological features of the reproductive tract.
Generation of Mice with Uterine Conditional Knockout of Ezh2
Control (Ezh2flox/flox; Ctrl) and Ezh2 conditional knockout (Ezh2flox/flox; PgrCre/+; Ezh2 cKO) mice were generated. The genotype of mice was analyzed by genomic PCR based on an established The Jackson Laboratory protocol using mouse tail DNA and primers Ezh2 flox-forward (5′-CATGTGCAGCTTTCTGTTCA-3′) and Ezh2 flox-reverse (5′-CACAGCCTTTCTGCTCACTG-3′) (wild-type band = 203 bp, and flox band = approximately 300 bp). The primers used to amplify the recombined/mutant Ezh2 band (200 bp) were 5′-CCCATGTTTAAGGGCATAGTGACATG-3′ (forward) and 5′-TCGAGGGACCTAATAACTCGTATAGCA-3′ (reverse).22 Hypoxanthine guanine phosphoribosyltransferase (Hprt) was used as an internal control.24 Genotyping of Pgr-Cre and analysis of PCR products were described elsewhere.25, 26
Immunohistochemistry and Immunofluorescence
Immunostaining was detailed previously.26, 27 Briefly, paraffin sections (5 μm thick) were deparaffinized, rehydrated, and boiled in citrate buffer (pH = 6) to expose the antigen, followed by incubation with 3% H2O2 solution for 10 minutes. Inactivation of endogenous peroxidase activity was only performed for the immunohistochemical procedure. Sections were blocked before incubation with primary antibodies (Table 1) overnight at 4°C. After primary antibody incubation, the avidin-biotin complex kit (catalog number PK-6100; Vector Laboratories, Burlingame, CA) was used, with NovaRed (catalog number SK-4800; Vector Laboratories) being the developing substrate (for immunohistochemistry). Sections were mounted using mounting media [catalog number 17986-01 (Thermo Fisher Scientific, Waltham, MA) or catalog number 3801743 (Leica Biosystems, Buffalo Grove, IL)]. For immunofluorescence microscopy, sections were mounted using medium that contains DAPI, a nuclear counterstain (catalog number P36931; Thermo Fisher Scientific).
Table 1.
Information of Primary Antibodies
| Name | Manufacturer | Catalog number | Host | IHC/IF |
|---|---|---|---|---|
| EZH2∗ | Cell Signaling (Danvers, MA) | 5246 | Rabbit | 1:400 |
| FOXA2 | Abcam (Cambridge, MA) | Ab108422 | Rabbit | 1:250 |
| Ki-67 | Abcam | Ab16667 | Rabbit | 1:200 |
| VIM | Cell Signaling | 5741 | Rabbit | 1:200 |
| ACTA2 | Cell Signaling | 19245 | Rabbit | 1:500 |
| KRT8 | DSHB (Iowa City, IA) | TROMA-I | Rat | 1:200 |
| KRT14 | Thermo Fisher Scientific (Waltham, MA) | PA5-16722 | Rabbit | 1:400 |
| KRT14 | Santa Cruz Biotechnology (Dallas, TX) | SC-53253 | Mouse | 1:100 |
| KRT5 | BioLegend (San Diego, CA) | 905501 | Rabbit | 1:3000 |
| KRT6A | BioLegend | 905701 | Rabbit | 1:1000 |
| ΔNp63 | BioLegend | 619001 | Rabbit | 1:500 |
ACTA2, α-smooth muscle actin; DSHB, Developmental Studies Hybridoma Bank; EZH2, enhancer of zeste 2 polycomb repressive complex 2 subunit; FOXA2, forkhead box A2; IF, immunofluorescence; IHC, immunohistochemistry; KRT, cytokeratin; ΔNp63, tumor protein 63 not containing an N-terminal transactivation domain; VIM, vimentin.
Western blot analysis: 1:1000
Dolichos Biflorus Agglutinin Lectin Staining
Dolichos biflorus agglutinin staining was performed, as detailed elsewhere, with slight modifications.28 After deparaffinization and rehydration, sections were boiled in citrate buffer (pH 6) for 20 minutes, blocked with 5% bovine serum albumin in Tris-buffered saline for 30 minutes, and incubated with fluorescein-labeled dolichos biflorus agglutinin (catalog number FL-1031; Vector Laboratories), that was diluted 1:100 in 1% bovine serum albumin/Tris-buffered saline overnight at 4°C. Sections were mounted with DAPI-containing medium described above.
Western Blot Analysis
Uterine protein samples were prepared, and Western blot analysis was conducted, as previously described.26 Information for primary antibodies is listed in Table 1. Chemiluminescent substrate (catalog number WBKLS0100; Millipore Sigma) was used for signal detection. Digital images were captured using the ChemiDoc imaging system (Bio-Rad, Hercules, CA) and processed using Adobe Photoshop CS5 (Adobe Inc., San Jose, CA).
Quantitative RT-PCR
RNA isolation and quantitative RT-PCR were conducted as described.29 Reverse transcription was performed using 500 ng (for uterine or decidual tissues) or 200 ng (for uterine epithelium) of total RNA and Superscript III Reverse Transcriptase (catalog number 18080044; Thermo Fisher Scientific). Quantitative PCR was performed in duplicate using either SYBR Green (catalog number 1725121; Bio-Rad) or TaqMan Mix (catalog number 4304437; Thermo Fisher Scientific). The sequences of gene-specific primers3, 22, 25, 27, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 and information on TaqMan probes are listed in Table 2. The average CT value for each gene was normalized to that of ribosomal protein L19 (Rpl19), and relative gene expression levels were calculated as described.43
Table 2.
Real-Time RT-PCR Primers
| Primers used for SYBR Green assays | |||
|---|---|---|---|
| Name | Sequence | Reference | |
| Ezh2del | Forward | 5′-TTACTGCTGGCACCGTCTGATGTG-3′ | 22 |
| Reverse | 5′-TGTCTGCTTCATCCTGAGAAATAATCTCC-3′ | ||
| Foxa2 | Forward | 5′-AGCAGAGCCCCAACAAGA-3′ | 3 |
| Reverse | 5′-AGAGAGAGTGGCGGATGGAG-3′ | ||
| Krt8 | Forward | 5′-TCCATCAGGGTGACTCAGAAA-3′ | PrimerBank ID 52789a1∗ |
| Reverse | 5′-CCAGCTTCAAGGGGCTCAA-3′ | ||
| Krt14 | Forward | 5′-GCTGGATGTGAAGACAAGGCTGGAG-3′ | 3 |
| Reverse | 5′-ATTGGGAAGATGAAAGGTGG-3′ | ||
| Krt17 | Forward | 5′-TCCCAGCTCAGCATGAAAGC-3′ | 30 |
| Reverse | 5′-CTTGTACTGAGTCAGGTGGGC-3′ | ||
| TAp63 | Forward | 5′-GTGGATGAACCTTCCGAAAA-3′ | 31 |
| Reverse | 5′-GAGGAGCCGTTCTGAATCTG-3′ | ||
| ΔNp63 | Forward | 5′-GAGCAGCCTTGACCAGTCTC-3′ | 31 |
| Reverse | 5′-GAGGAGCCGTTCTGAATCTG-3′ | ||
| Fgfr2 | Forward | 5′-CCTCGATGTCGTTGAACGGTC-3′ | PrimerBank ID 198594a1∗ |
| Reverse | 5′-CAGCATCCATCTCCGTCACA-3′ | ||
| Wnt4 | Forward | 5′-CATCGAGGAGTGCCAATACCA-3′ | 32 |
| Reverse | 5′-GGAGGGAGTCCAGTGTGGAA-3′ | ||
| Wnt7a | Forward | 5′-CGACTGTGGCTGCGACAAG-3′ | 33 |
| Reverse | 5′-CTTCATGTTCTCCTCCAGGATCTTC-3′ | ||
| Pten | Forward | 5′-ACACCGCCAAATTTAACTGC-3′ | 34 |
| Reverse | 5′-TACACCAGTCCGTCCCTTTC-3′ | ||
| Bmp2 | Forward | 5′-GGGACCCGCTGTCTTCTAGT-3′ | 35 |
| Reverse | 5′-TCAACTCAAATTCGCTGAGGAC-3′ | ||
| Il15 | Forward | 5′-ACATCCATCTCGTGCTACTTGT-3′ | 25 |
| Reverse | 5′-GCCTCTGTTTTAGGGAGACCT-3′ | ||
| Klrg1 | Forward | 5′-TTTGGGGCTTTTGACTGTGAT-3′ | 25 |
| Reverse | 5′-TGTAAGGAGATGTGAGCCTTTGT-3′ | ||
| Prf1 | Forward | 5′-TCTCCTCCTATGGCACGCAC-3′ | 25 |
| Reverse | 5′-TGTAAGGACCGAGATGCGG-3′ | ||
| Bmpr2 | Forward | 5′-TTGGGATAGGTGAGAGTCGAAT-3′ | 41 |
| Reverse | 5′-TGTTTCACAAGATTGATGTCCCC-3′ | ||
| Tgfbr1 | Forward | 5′-TGCCATAACCGCACTGTCA-3′ | 27 |
| Reverse | 5′-AATGAAAGGGCGATCTAGTGATG-3′ | ||
| Vegfa | Forward | 5′-AGACAGAACAAAGCCAGAAAATCA-3′ | 42 |
| Reverse | 5′-AATGCTTTCTCCGCTCTGAA-3′ | ||
| Angpt2 | Forward | 5′-GATCTTCCTCCAGCCCCTAC-3′ | 25 |
| Reverse | 5′-TTTGTGCTGCTGTCTGGTTC-3′ | ||
| Wnt6 | Forward | 5′-GCGGAGACGATGTGGACTTC-3′ | 36 |
| Reverse | 5′-ATGCACGGATATCTCCACGG-3′ | ||
| Sfrp4 | Forward | 5′-AGAAGGTCCATACAGTGGGAAG-3′ | 37 |
| Reverse | 5′-GTTACTGCGACTGGTGCGA-3′ | ||
| Dact1 | Forward | 5′-TCAGGGTTTTATGAGCTGAGT-3′ | 38 |
| Reverse | 5′-GAACACGGAGTTGGAGGAGTTA-3′ | ||
| Mtor | Forward | 5′-ACCGGCACACATTTGAAGAAG-3′ | PrimerBank ID 9910228a1∗ |
| Reverse | 5′-CTCGTTGAGGATCAGCAAGG-3′ | ||
| Ptgs2 | Forward | 5′-TGAGCAACTATTCCAAACCAGC-3′ | 39 |
| Reverse | 5′-GCACGTAGTCTTCGATCACTATC-3′ | ||
| Hoxa10 | Forward | 5′-CCTGCCGCGAACTCCTTTT-3′ | PrimerBank ID 6680243a1∗ |
| Reverse | 5′-GGCGCTTCATTACGCTTGC-3′ | ||
| Fkbp3 | Forward | 5′-TTGCCAAGACTGCTAATAAGGAC-3′ | PrimerBank ID 7305061a1∗ |
| Reverse | 5′-TTGGTGGACCCTCATCAAGAG-3′ | ||
| Fkbp4 | Forward | 5′-CCTCTCGAAGGAGTGGACATC-3′ | PrimerBank ID 6753882a1∗ |
| Reverse | 5′-TCCCCGATCATGGGTGTCT-3′ | ||
| Fkbp5 | Forward | 5′-TGAGGGCACCAGTAACAATGG-3′ | PrimerBank ID 6753884a1∗ |
| Reverse | 5′-CAACATCCCTTTGTAGTGGACAT-3′ | ||
| Rpl19 | Forward | 5′-ATGAGTATGCTCAGGCTACAGA-3′ | 29 |
| Reverse | 5′-GCATTGGCGATTTCATTGGTC-3′ | ||
| TaqMan gene expression assay | ||
|---|---|---|
| Name | ID | Manufacturer |
| Ezh1 | Mm01292494_g1 | Thermo Fisher Scientific |
| Krt5 | Mm00503549_m1 | Thermo Fisher Scientific |
| Prl8a2 | Mm01135453_m1 | Thermo Fisher Scientific |
| Corin | Mm00444120_m1 | Thermo Fisher Scientific |
| Rpl19 | Mm02601633_g1 | Thermo Fisher Scientific |
ID, identification.
Accessible at https://pga.mgh.harvard.edu/primerbank.
Statistical Analysis
The statistical difference between two groups was examined using the unpaired two-tailed t-test. Data are given as means ± SEM. P < 0.05 denotes statistical significance.
Results
Conditional Ablation of EZH2 in the Uterus
Expression of EZH2 has been reported in the glandular epithelium and stroma of the human uterus.16 To verify the expression of EZH2 in the mouse uterus, immunohistochemistry was performed using an antibody directed to EZH2. The results showed strong immunoreactive signals of EZH2 in both luminal and glandular epithelia of the mouse uterus (Supplemental Figure S1). Lower levels of EZH2 were detected in the myometrium (Supplemental Figure S1). Immunostaining for EZH2 in the stroma appeared to be variable during the estrous cycle (Supplemental Figure S1).
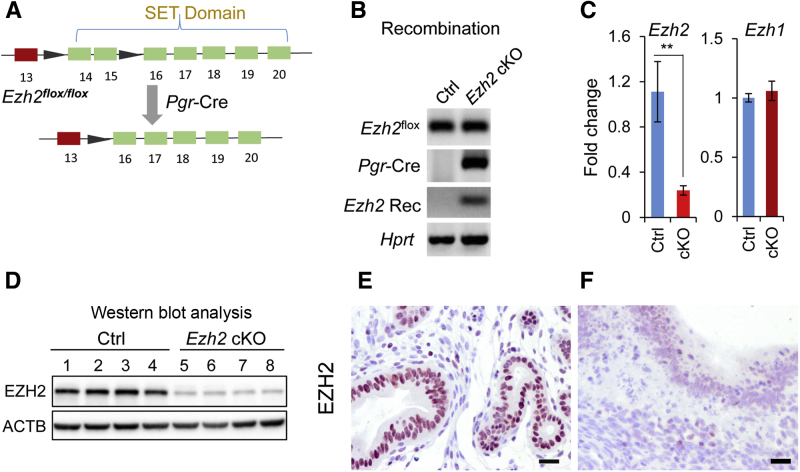
EZH2 is involved in epithelial-mesenchymal transition in cancer cells.44 To determine whether EZH2 is required for uterine epithelial integrity, uterine Ezh2 was deleted using Pgr-Cre that is expressed in both epithelial and mesenchyme-derived cells of the uterus.21 Figure 1A depicts the conditional allele of Ezh2 in which exons 14 and 15 are flanked by two locus of X-over of P1 (LoxP) sites.22 Cre-mediated deletion of the target exons is expected to cause a frameshift mutation that eliminates the SET domain.22 As anticipated, deletion of Ezh2 was evidenced by the presence of a recombined Ezh2 allele in the Ezh2 cKO uterus (Figure 1, A and B). Consistent with this, real-time PCR detected a significant reduction of Ezh2, but not Ezh1, mRNA levels in 1-month–old Ezh2 cKO mice (Figure 1C), confirming Ezh2-specific deletion in the uterus. Ablation of EZH2 was further verified by Western blot analysis (Figure 1D) and immunohistochemical analyses using uteri from 2-month–old mice (Figure 1, E and F). Thus, EZH2 was efficiently depleted in the uterus.
Figure 1.
Conditional deletion of Ezh2 in the mouse uterus. A: Schematic representation of Pgr-Cre–mediated inactivation of Ezh2 floxed allele. B: Recombination of Ezh2 conditional allele in Ezh2 cKO uteri. The Ezh2 recombined allele (Ezh2 Rec) was only detectable in the uterus of the Ezh2 cKO mouse but not the control (Ctrl). C: Reduction of Ezh2, but not Ezh1, mRNA levels in the uteri of 1-month–old Ezh2 cKO mice versus controls. D: Western blot analysis of EZH2 protein levels in control and Ezh2 cKO mice. Each lane represents an independent uterine sample from a 2-month–old mouse. β-Actin (ACTB) was included as an internal control. E and F: Immunohistochemical staining of EZH2 in the uteri of 2-month–old control and Ezh2 cKO mice. Three independent samples per group were examined. Note the diminished immunoreactive signals in the epithelia of Ezh2 cKO uteri (F) compared with controls (E). Data are expressed as means ± SEM (C). n = 4 Ctrl mice (C and D); n = 5 cKO mice (C); n = 4 cKO mice (D). ∗∗P < 0.01. Scale bars = 20 μm (E and F).
Depletion of EZH2 Leads to the Formation of Stratified Uterine Epithelium
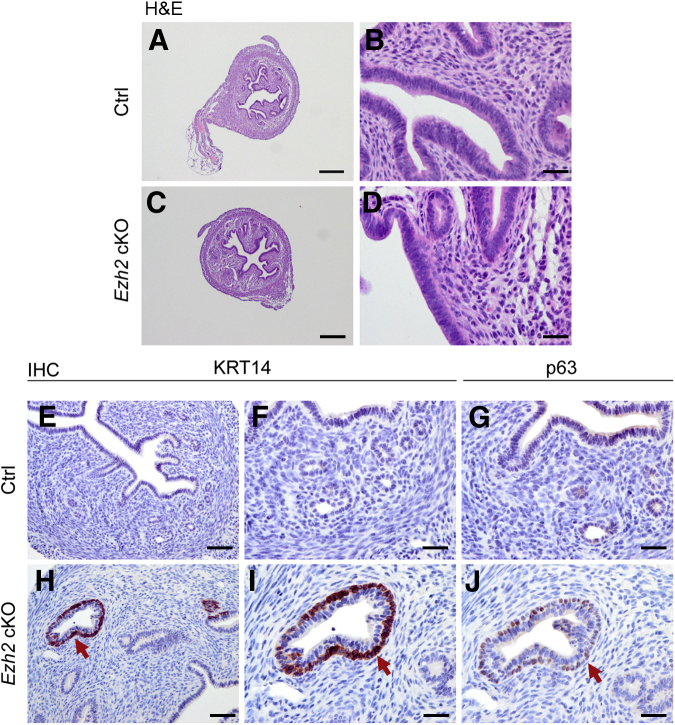
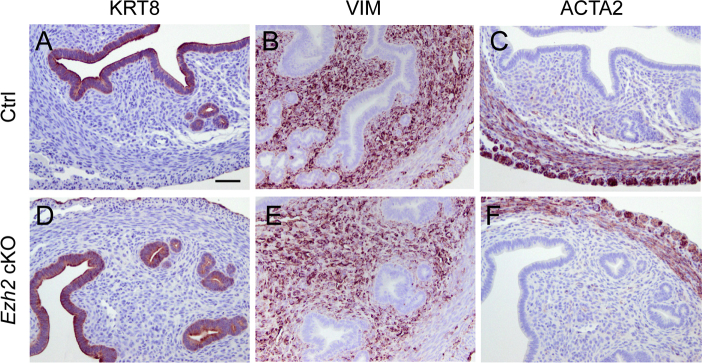
To determine the potential impact of EZH2 depletion on uterine development, we examined the morphology of the uterus at 1 month of age. The uterus was histologically normal, consisting of major structural components, including the myometrium and endometrium, revealed by hematoxylin and eosin staining (Figure 2, A–D). It is well established that cytokeratin 8 (KRT8) is expressed in simple epithelia, whereas KRT5, KRT6, and KRT14 are produced by stratified epithelia.45 Immunostaining of KRT8 showed the presence of both luminal and glandular epithelium within the endometrium (Supplemental Figure S2, A and D). Both Ezh2 cKO and control uteri expressed stromal cell marker vimentin (Supplemental Figure S2, B and E). The myometrium of Ezh2 cKO mice appeared to be indistinguishable from that of controls by immunostaining of α-smooth muscle actin (Supplemental Figure S2, C and F). However, further immunohistochemical analysis of the uterus, using antibodies directed to basal cell markers KRT14 and tumor protein 63 (p63), demonstrated focal expression of these proteins in a cell layer beneath luminal and/or glandular epithelial cells in Ezh2 cKO uteri (Figure 2, H–J), which is in sharp contrast to normal controls (Figure 2, E–G).
Figure 2.
Ablation of EZH2 results in focal formation of stratified uterine epithelium. A–D: Hematoxylin and eosin staining of uterine samples from 1-month–old control (Ctrl) and Ezh2 cKO mice. A–D: Higher-magnification images (B and D) for A and C, respectively. E–J: Immunostaining of cytokeratin 14 (KRT14) and tumor protein 63 (p63) using 1-month–old control and Ezh2 cKO uteri. Note the immunoreactive signals of KRT14 beneath the epithelial layer of a uterine gland and the p63-specific signals in the same gland on an adjacent section (arrows). E, F, H, and I: Higher-magnification images (F and I) for E and H, respectively. Three independent samples per group were examined. Scale bars: 250 μm (A and C); 25 μm (B, D, F, G, I, and J); 50 μm (E and H). Original magnification: ×40 (A and C); ×400 (B, D, F, G, I, and J); ×200 (E and H). H&E, hematoxylin and eosin staining; IHC, immunohistochemistry.
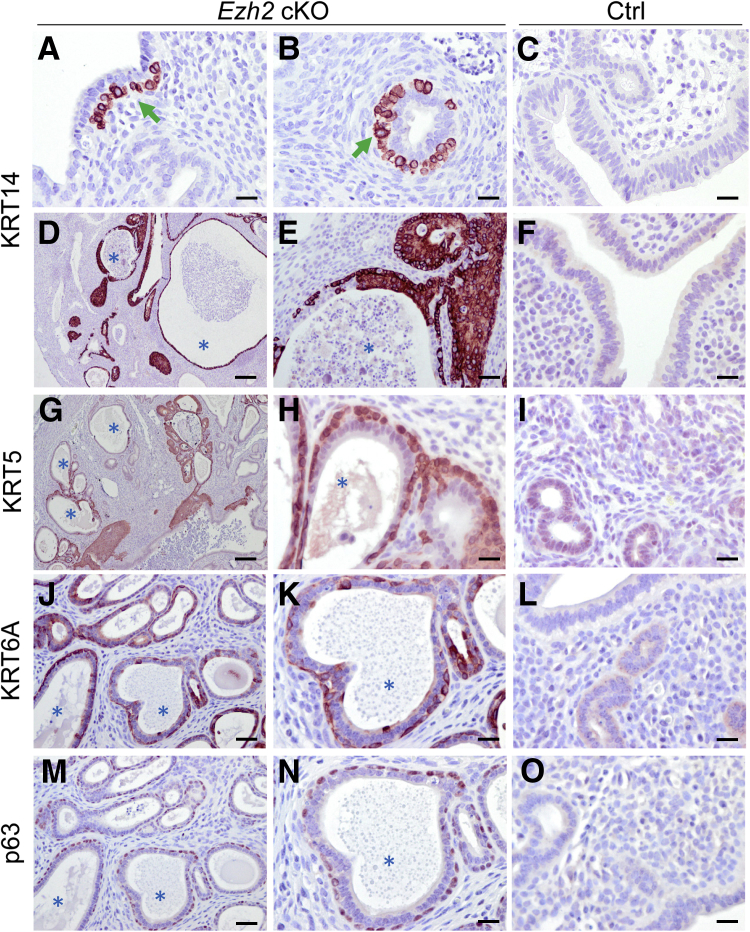
Similar epithelial abnormalities in limited regions were found in the uteri of 2-month–old Ezh2 cKO mice (Figure 3, A and B) compared with controls (Figure 3C). At approximately 8 months of age, epithelial stratification was escalated in Ezh2 cKO mice. This was evidenced by the presence of cells positively stained for KRT14 (Figure 3, D and E), KRT5 (Figure 3, G and H), KRT6A (Figure 3, J and K), and p63 (Figure 3, M and N) in the uteri of Ezh2 cKO mice. Age-matched controls were included (Figure 3, F, I, L, and O). The development of stratified epithelia was accompanied by endometrial hyperplasia, evident in nulliparous Ezh2 cKO mice at the age of 5 to 8 months (Figure 3 and Supplemental Figure S3). Histologically, cystic dilation of endometrial glands containing eosin-stained substance could be observed in Ezh2 cKO uteri (Supplemental Figure S3, B and C), compared with controls (Supplemental Figure S3A). The morphologically altered glands were variable in size, and some of them contained stratified epithelial layers (Figure 3, D, E, G, H, J, K, M, and N, and Supplemental Figure S3, B and C), with nuclear atypia in some cases (Figure 3, E and H, and Supplemental Figure S3, C and D). A minor level of gland crowding was observed in discrete regions of Ezh2 cKO uteri, in which intervening stroma among glands was sparse (Supplemental Figure S3, B and D). Immunostaining of vimentin that marks uterine stroma confirmed this observation (Supplemental Figure S3, E and F). Despite the pathologic changes in the endometrium, the myometrial layers remained intact in Ezh2 cKO mice (Supplemental Figure S3B). Moreover, immunoreactive signals for Ki-67 could be detected in some abnormal glands or cystic glandular structures in Ezh2 cKO uteri (Supplemental Figure S3, G–L). The weight of uterine horns from Ezh2 cKO mice (28.8 ± 5.6 mg; n = 3) was increased compared with controls (11.7 ± 1.1 mg; n = 6), even before sexual maturity (1 month; P < 0.01). Collectively, the endometrial pathology in Ezh2 cKO mice resembled human endometrial hyperplasia.46, 47
Figure 3.
Epithelial stratification and endometrial hyperplasia in aged Ezh2 cKO mice. A–F: Expression of cytokeratin 14 (KRT14) in the uteri from control (Ctrl) and Ezh2 cKO mice at 2 (A–C) and 8 (D–F) months of age. More extensive immunoreactive signals of KRT14 were found in the uteri of 8-month–old mice (D and E) versus 2-month–old mice (A and B). Panel E shows a higher-magnification image of D. Arrows indicate KRT14-positive cells. G–O: Expression of KRT5, KRT6A, and tumor protein 63 (p63) in 8-month–old Ezh2 cKO and control mice. G, H, J, K, M, and N: Higher-magnification images (H, K, and N) for G, J, and M, respectively. J, K, M, and N: Adjacent sections (M and N) for J and K, respectively. At least three independent samples per group were examined. Asterisks indicate cyst-like structures. Scale bars: 20 μm (A–C, F, H, I, K, L, N, and O); 40 μm (E, J, and M); 200 μm (D and G).
To determine whether the observed epithelial abnormalities in the EZH2-ablated uterus were associated with dysregulation of genes known to regulate uterine epithelial stratification, the transcript levels of Fgfr2,3 wingless-type MMTV integration site family member 4 (Wnt4),48 Wnt7a,49 and phosphatase and tensin homolog (Pten)50 were examined using both uterine tissues and isolated uterine epithelia from Ezh2 cKO mice. Significant differences were not detected in the expression of these genes in uterine tissues or epithelial preparations between control and Ezh2 cKO mice (Supplemental Figure S4).
Altered FOXA2 Expression in EZH2-Depleted Uterus
A recent study has shown that altered expression of FOXA2 is associated with epithelial stratification.51 Therefore, it was determined if dysregulation of FOXA2 contributed to the formation of stratified epithelia in Ezh2 cKO uteri. At 2 months of age, glandular epithelia were identified by FOXA2 staining in control uteri (Figure 4, A and B). However, partial loss of FOXA2 immunostaining was observed in the glandular epithelia of Ezh2 cKO mice (Figure 4, C–F). This result agrees with the finding that Foxa2 mRNA levels were decreased in uterine epithelia of Ezh2 cKO mice at 1 month of age (Figure 4G). In addition, double immunofluorescence staining of KRT14 and FOXA2 showed no FOXA2 expression in KRT14-positive epithelial cells lining the luminal/glandular epithelia of Ezh2 cKO uteri (Figure 5, B, D, and F), in contrast to controls lacking specific KRT14 staining (Figure 5, A, C, and E). Negative controls are shown in Figure 5, G and H. Because FOXA2 regulates the expression of genes involved in uterine gland development and function,52 these results suggest that conditional deletion of Ezh2 may impair uterine function.
Figure 4.
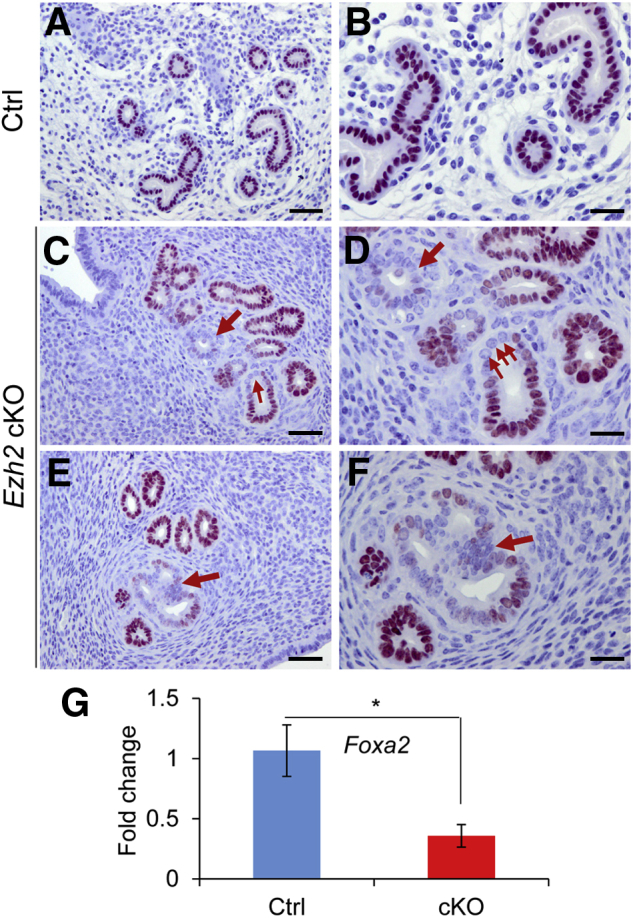
Dysregulation of forkhead box A2 (FOXA2) in the uteri of Ezh2 cKO mice. A–F: Immunohistochemical analysis of FOXA2 distribution in the uteri of control (Ctrl) and Ezh2 cKO mice at the age of 2 months. A, C, and E are shown at higher magnification in B, D, and F, respectively. Note the reduction or partial loss of FOXA2 signals in some uterine glands of Ezh2 cKO mice versus controls (arrows). Three independent samples per group were examined. G: Real-time PCR analysis of expression of Foxa2 mRNA in uterine epithelia isolated from control and Ezh2 cKO mice. Data are expressed as means ± SEM (G). n = 4 (G). ∗P < 0.05. Scale bars: 50 μm (A, C, and E); 25 μm (B, D, and F). Original magnification: ×200 (A, C, and E); ×400 (B, D, and F).
Figure 5.
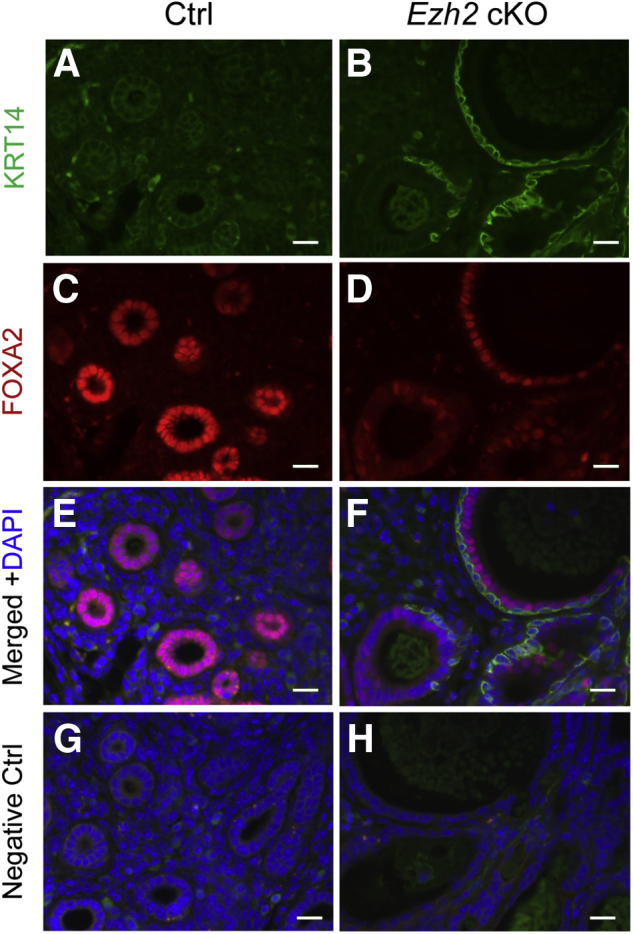
Minimal forkhead box A2 (FOXA2) expression in cytokeratin 14 (KRT14)–positive cells. A–F: Double-immunofluorescence staining of KRT14 and FOXA2 in the uteri of control (Ctrl; A, C, and E) and Ezh2 cKO (B, D, and F) mice at the age of 8 months. KRT14-positive cells (green) did not express FOXA2 (red). G and H: Negative controls, using isotype-matched IgGs to replace primary antibodies, are shown. At least three independent samples per group were examined. Scale bars = 20 μm (A–H).
Conditional Deletion of EZH2 in the Mouse Uterus Produces Basal-Like Epithelial Cells Expressing Genes Associated with the Basal Cell Phenotype
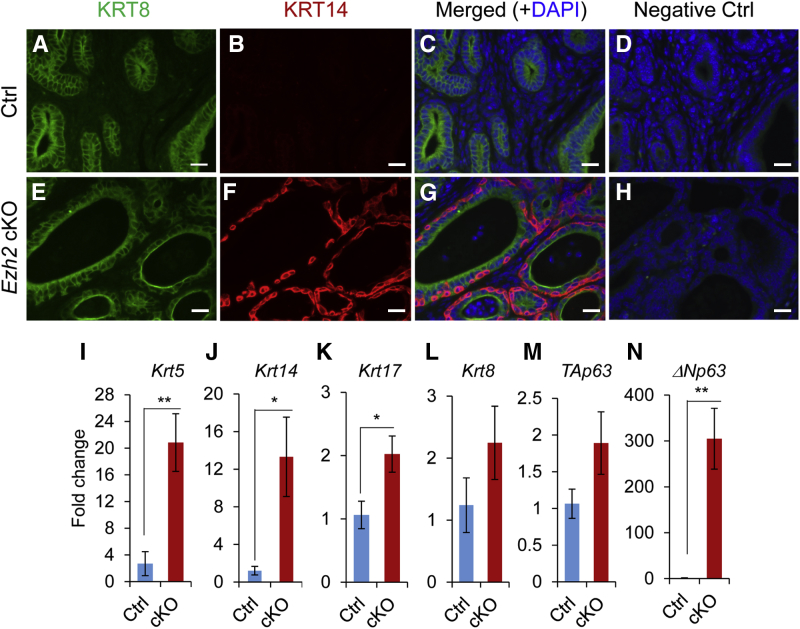
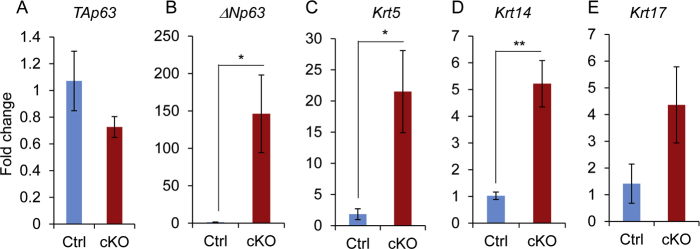
Double indirect immunofluorescence of KRT8 and KRT14 demonstrated the presence of basal-like epithelial cells (KRT14 positive but KRT8 negative) lining some KRT8-positive epithelial cells in the uteri of Ezh2 cKO mice (Figure 6, E–H), in contrast to the controls in which epithelia were only stained for KRT8 (Figure 6, A–D). To understand the molecular underpinnings of the observed basal-like cell phenotype, it was examined whether ablation of EZH2 promoted the expression of basal cell–related genes, including Krt5, Krt14, Krt17, and TAp63 and ΔNp63 [containing an N-terminal transactivation (TA) domain and not containing this domain, respectively], that encode p63 isoforms using 1-month–old mice. Results showed that transcript levels of Krt5, Krt14, and Krt17 were significantly elevated in Ezh2 cKO uteri compared with those in age-matched controls (Figure 6, I–K). In contrast, mRNA levels of Krt8 were not significantly altered between Ezh2 cKO and control uteri (Figure 6L). Interestingly, the mRNA expression of ΔNp63, but not TAp63, was dramatically increased in the uteri of Ezh2 cKO mice (Figure 6, M and N). To further demonstrate dysregulation of basal cell–related genes in the epithelial compartment, uterine epithelia were isolated from both control and Ezh2 cKO uteri at 1 month of age and real-time PCR analysis was performed. In line with results obtained using uterine tissues, increased expression of ΔNp63, Krt5, and Krt14 was found in uterine epithelia of Ezh2 cKO mice compared with controls (Supplemental Figure S5). Thus, loss of EZH2 produces basal-like epithelial cells or a cell population expressing basal markers.
Figure 6.
Ablation of EZH2 produces basal-like cell types with up-regulation of basal cell makers. A–H: Double-immunofluorescence staining of cytokeratin 8 (KRT8) and KRT14 in the uteri of control (Ctrl; A–D) and Ezh2 cKO (E–H) mice at the age of 8 months. D and H: Negative controls using IgGs are shown. At least three independent samples per group were examined. I–N: Real-time PCR analysis of Krt5, Krt14, Krt17, Krt8, TAp63, and ΔNp63 transcript levels in the uteri of control and Ezh2 cKO mice at the age of 1 month. Data are expressed as means ± SEM (I–N). n = 4 (I–N, Ctrl mice); n = 5 (I–N, cKO mice). ∗P < 0.05, ∗∗P < 0.01. Scale bars = 20 μm (A–H).
Ablation of EZH2 Impairs Female Fertility
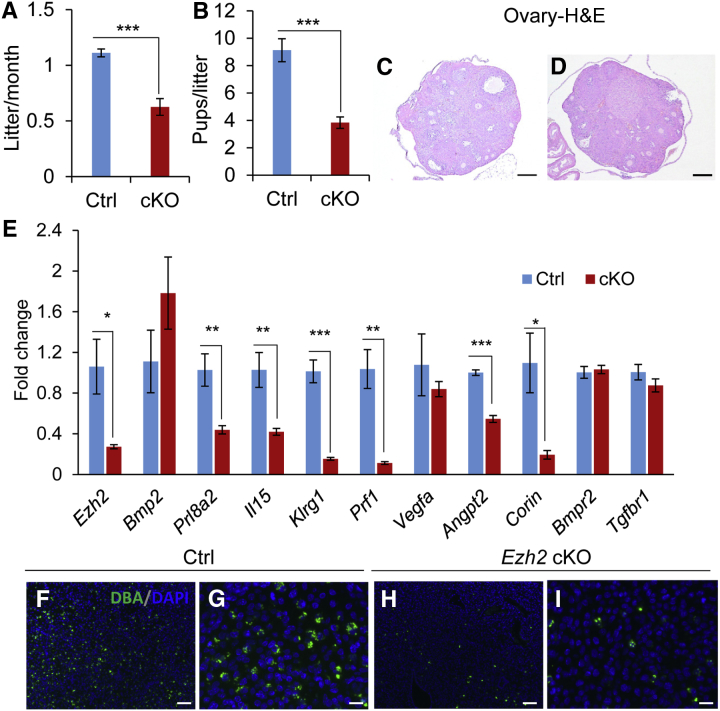
To examine whether EZH2 depletion in the uterus impacts female fertility, Ezh2 cKO mice and controls were continuously mated with fertile male mice for a period of 6 months. During the fertility test, Ezh2 cKO mice showed a reduced number of litters and a smaller litter size compared with controls (Figure 7, A and B). Although all control mice delivered normally, approximately 64% of Ezh2 cKO mice exhibited signs of dystocia or difficulty in labor during the testing period. Histologic analysis of the ovary showed normal follicular development in Ezh2 cKO mice (Figure 7, C and D). Furthermore, blastocysts could be retrieved from Ezh2 cKO uteri at embryonic day 3.5 (E3.5; data not shown). Our findings indicate that the reduced reproductive potential of Ezh2 cKO mice is likely caused by impaired uterine function.
Figure 7.
Reproductive defects in Ezh2 cKO mice. A and B: Reduced number of litters and litter size during a 6-month fertility test. Approximately 64% of Ezh2 cKO mice, but not controls (Ctrls), developed signsed of dystocia or difficulty in labor during the fertility test. In that case, fertility parameters were calculated on the basis of data collected before mice developed the labor complication. C and D: Hematoxylin and eosin staining of ovaries from control and Ezh2 cKO mice at 2 months of age. E: Real-time PCR analysis of expression of genes associated with decidual differentiation, uterine natural killer (uNK) cells, and transforming growth factor-β family signaling in embryonic day 8.5 (E8.5) decidual tissues from control and Ezh2 cKO mice. F–I: Reduction of uNK cells in E8.5 decidual basalis of Ezh2 cKO mice, evidenced by dolichos biflorus agglutinin (DBA) staining. F–I: F and H are shown at higher magnification in G and I, respectively. Data are expressed as means ± SEM (A, B, and E). n = 11 (A and B, cKO mice); n = 6 (A and B, Ctrl mice); n = 3 (C, D, E, Ctrl mice, and F–I); n = 4 (E, cKO mice). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Scale bars: 250 μm (C and D); 100 μm (F and H); 25 μm (G and I). Original magnification: ×40 (C and D); ×100 (F and H); ×400 (G and I). H&E, hematoxylin and eosin staining.
To explore the potential effect of Ezh2 deletion on uterine decidual function, it was first verified that Ezh2 expression was reduced in E8.5 decidual tissues collected from Ezh2 cKO mice (Figure 7E). Implantation sites could be found in Ezh2 cKO mice at E8.5 (Supplemental Figure S6A), suggesting that decidualization occurred in these mice. Further analysis revealed potential defects in decidual differentiation, evidenced by impaired expression of prolactin family 8 subfamily a member 2 (Prl8a2), a decidual marker, in Ezh2 cKO decidual tissues (Figure 7E). However, mRNA expression of another key regulator of decidualization, bone morphogenetic protein 2 (Bmp2), was not significantly altered (Figure 7E). The analysis was extended to additional genes involved in decidualization, including Wnt4, Wnt6, secreted frizzled-related protein 4 (Sfrp4), disheveled-binding antagonist of β-catenin 1 (Dact1), mechanistic target of rapamycin kinase (Mtor; alias Frap1), prostaglandin-endoperoxide synthase 2 (Ptgs2), homeobox A10 (Hoxa10), and FK-506–binding protein 3 (Fkbp3), Fkbp4, and Fkbp5.48, 53, 54, 55 None of these genes was significantly altered, except Fkbp4, which was up-regulated in Ezh2 cKO uteri (Supplemental Figure S6B). Because decidualization is accompanied by active angiogenesis and vascular remodeling,56, 57 the expression of angiogenic factors, including vascular endothelial growth factor A (Vegfa), angiopoietins (Angpts) 1, 2, and 4, and Corin, a gene critical for uterine spiral artery remodeling, was next examined.58 The transcript levels of Angpt2 and Corin were significantly reduced in E8.5 deciduae from Ezh2 cKO mice (Figure 7E). Interestingly, dysregulation of genes associated with the recruitment/differentiation of uterine natural killer cells, including IL-15 (Il-15), killer cell lectin-like receptor subfamily G member 1 (Klrg1), and perforin 1 (Prf1), was also found (Figure 7E). Dolichos biflorus agglutinin lectin staining showed that the number of uterine natural killer cells was reduced in E8.5 decidual basalis of Ezh2 cKO mice versus controls (Figure 7, F–I). Because defects in uterine natural killer cell recruitment/differentiation were found in mice with conditional deletion of BMP receptor 2 (BMPR2) or transforming growth factor-β receptor 1 (TGFBR1),25, 59 the expression of both genes was examined in the deciduae of Ezh2 cKO mice. Results showed comparable expression levels of Bmpr2 and Tgfbr1 mRNA in decidual tissues between Ezh2 cKO mice and controls (Figure 7E), suggesting that EZH2 may act independently or upstream/downstream of BMPR2 and TGFBR1. The findings of dysregulation of genes involved in angiogenesis and vascular remodeling, together with the observed reduction of the number of uterine natural killer cells in Ezh2 cKO mice, indicate potentially impaired decidual function during pregnancy.
Discussion
Numerous reports highlight the involvement of EZH2 in multiple stages of cancer development, including initiation, formation, progression, and metastasis.60 As a core member of the PRC2 group, EZH2 promotes the proliferation and invasion of endometrial carcinoma,61 drives epithelial-mesenchymal transition in endometriosis,62 and hampers the expression of DNA damage repair genes in uterine fibroids.15 In contrast, the physiological function of EZH2 in the uterus remains largely unknown. One study using in vitro cultured human endometrial stromal cells indicates that decidualization is accompanied by a down-regulation of EZH2 expression to allow for epigenetic programming.16 Treatment of mice with DNA demethylating agent, 5-aza-2′-deoxycytidine, impairs embryo implantation.63 These studies indicate that modifications of chromatin and DNA are important for normal uterine function. Global deletion of Ezh2 leads to embryonic lethality during early development,64 precluding further characterization of the function of EZH2 during postnatal uterine development. This issue was circumvented by using the Cre-LoxP approach to delete Ezh2 in the uterus. EZH2 seems to play important roles in maintaining endometrial homeostasis and integrity.
The inner surface of the uterus is lined by a single layer of simple columnar epithelial cells, which extend into the endometrium to form uterine glands. Stratification of uterine epithelial cells is a pathologic event and has been observed in several genetically modified mouse models described below. Conditional deletion of Fgfr2 in the mouse uterus leads to the formation of stratified luminal epithelium, with the occurrence of p63 and KRT14-positive basal cells.3 Despite the normal appearance of uterine stromal cells and the myometrial layer, these mice show fertility defects due to pregnancy loss during the peri-implantation period.3 Loss of PTEN in the mouse uterus, using Pgr-Cre, promotes tumorigenesis, epithelial stratification, and squamous differentiation, characterized by the presence of KRT5- and p63-positive epithelial cells.50 In contrast, deletion of Pten, specifically in the epithelium using lactoferrin-Cre, does not cause stratification but results in apoptosis of epithelial cells, suggesting a potential role of PTEN-mediated epithelial-mesenchymal communication in epithelial stratification.50 Dysregulation of the WNT pathway is also associated with epithelial stratification. Deletion of Wnt4 in the mouse uterus causes the formation of stratified luminal epithelium and glandular defects, accompanied by defective embryo implantation and stromal cell function.48 Wnt7a-null mice also show stratified epithelium and defective adenogenesis in the uterus.49 Interestingly, conditional deletion of Wnt7a in the mouse uterus postnatally does not induce stratification of uterine epithelium, suggesting that the timing of WNT7A loss may contribute to the phenotypic difference observed between the ubiquitous deletion and conditional knockout models.65 Further supporting the role of the WNT pathway in maintaining epithelial integrity, dysregulation of beta catenin induces epithelial stratification.66 Recently, it was shown that conditional overexpression of FOXA2 in the mouse uterus results in the development of stratified luminal/glandular epithelium.51 In the stratified luminal epithelium of adult transgenic mice, FOXA2 was expressed in the nuclei of cells within the upper nonbasal layer.51 These results indicate that dysregulation of FOXA2 may cause epithelial stratification.51 The observation that decreased FOXA2 expression in the uteri of adult Ezh2 cKO females was not accompanied by depletion of uterine glands suggests a stage-specific function of FOXA2 in uterine gland development. Indeed, a recent study showed that adenogenic defects in Foxa2 conditional knockout mice are dependent on the stage of FOXA2 depletion; and ablation of FOXA2 in the uteri of adult mice does not affect uterine gland formation.8 As FOXA2 is a key regulator of the function of uterine glands,8 reduced FOXA2 expression in Ezh2 cKO mice likely leads to impaired glandular function.
Despite these findings, little is known about how dysregulation of the aforementioned genes leads to epithelial stratification. Our results did not reveal a direct link between EZH2 and dysregulation of Fgfr2, Wnt4, Wnt7a, and Pten in the formation of stratified uterine epithelium. Instead, our data suggest that EZH2 is required for uterine epithelial integrity via suppressing the expression of p63, which is consistent with a role of EZH2 in restricting the basal cell lineage in the development of the lung.67, 68 p63 is a key gene implicated in the stratification of epithelium, as stratification is impaired and differentiation markers are absent in the skin of p63-deficient mice.69 In the mammary gland, overexpression of p63 in luminal cells reprograms them to the basal type.70 Because of the existence of alternative promoters, two classes of p63 proteins are translated (ie, TAp63 and ΔNp63).71 In addition, the alternative splicing at the C-terminus produces at least three isoforms (α, β, and γ).71 The exact function of p63 isoforms in epithelial stratification remains controversial. In the epidermis, TAp63 has been found to drive the stratification and expression of KRT5 and KRT14.72 However, other reports indicate that ΔNp63 may initiate stratification or expansion of the basal layer.73, 74, 75 In the uterus, the role of p63 isoforms in epithelial stratification remains unknown. The data support the involvement of ΔNp63 in uterine epithelial stratification in the absence of EZH2. On the basis of these findings, it is tempting to speculate that p63 and other basal cell genes are up-regulated as a result of decreased H3K27 methylation on loss of EZH2, leading to the differentiation of basal-like cells in the uterus. However, the function of these cells in Ezh2 cKO mice remains unclear. Because uterine epithelia generally do not contain basal cells, the appearance of this cell type and the expression of basal cell–associated keratins in EZH2-depleted uteri may indicate altered differentiation of epithelial cells that normally form a simple epithelium in the uterus. As basal-like cells in the uterus are associated with uterine dysfunction and tumor progression,3, 50 it is conceivable that epithelial stratification in Ezh2 cKO mice may link to the development of endometrial hyperplasia and/or reproductive deficiency.
Limitations exist for the current study. Because Pgr-Cre deletes genes in both epithelial and mesenchymal compartments, a potential contribution of epithelial-stromal interaction to the observed phenotype in Ezh2 cKO uteri should be considered. Future studies are needed to define how loss of EZH2 alters uterine growth and differentiation pathways and uncover the contribution of epithelial versus stromal EZH2 to the observed basal-like cell phenotype. Generation of Ezh2 conditional knockout mice using an epithelial- or stromal-specific Cre driver may help clarify this question. Ezh2 cKO female mice showed defects in parturition, the cause of which is unclear. Further investigations are warranted to gain mechanistic insights into this defect and determine whether EZH2 plays a role in the myometrium or cervix. It is also important to further define the epithelial versus stromal/decidual contribution to the observed reproductive defects. In addition, the methylation status of H3K27 of stratification-related genes (eg, p63, Krt14, and Krt5) in uterine epithelial cells of Ezh2 cKO mice versus controls was not determined because of technical challenges of chromatin immunoprecipitation–real-time quantitative PCR assays using low amounts of uterine epithelial cells isolated from mice at an early developmental stage or using uterine tissues that contain multiple cell types. Nevertheless, the current findings reveal a novel role for uterine EZH2 in reproductive development and function.
Understanding of the role of EZH2 and EZH1 and their potential redundancy in establishing H3K27 methylation and gene silencing is incomplete. Our results clearly showed an EZH1-independent role of EZH2 in the regulation of endometrial homeostasis and integrity and uterine function, supporting that EZH1 and EZH2 may play distinct roles in maintaining the repressive chromatin.76 However, several studies indicate that EZH2 is not the only histone methyltransferase that establishes the H3K27 methylation mark.22, 77, 78 Instead, EZH1 and EZH2 function redundantly in regulating the methylation status of H3K27 during development.22, 77, 78 Generation and characterization of mice with compound loss of EZH2 and EZH1 in the uterus will help determine the functional overlap between the two genes, if any, and elucidate the role of histone modifications in the uterus.
In summary, results from the current study suggest that EZH2 maintains uterine epithelial integrity, partially through inhibiting the differentiation of basal-like cells and the development of endometrial hyperplasia. More important, the altered uterine growth and impaired fertility in the Ezh2 cKO mouse make it a useful model to understand mechanisms regulating endometrial homeostasis and uterine function. Our findings support the concept that manipulation of epigenetic regulators could offer an exquisite approach to treat reproductive diseases and fertility issues related to epigenetic changes or epigenetic drift, caused by environmental or intrinsic cues.
Acknowledgments
We thank Dr. Yang Gao (Baylor College of Medicine) for setting up the initial mouse breeding colony.
X.F. and N.N. performed the experiments and analyzed the data; J.P.L. generated and provided Pgr-Cre mice; I.I., K.J.B., M.R., and Q.L. designed and supervised the work and offered guidance; X.F. and Q.L. wrote the manuscript; all authors provided valuable feedback on the final manuscript.
Footnotes
See related Commentary on page 1176
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH grant R01HD087236 (Q.L.) and a Texas A&M University T3 grant (Q.L.).
Disclosures: None declared.
Supplemental material for this article can be found at http://doi.org/10.1016/j.ajpath.2019.02.016.
Supplemental Data
Supplemental Figure S1.
Localization of EZH2 in the mouse uterus during the estrous cycle. Immunohistochemical staining of EZH2 using uterine samples from wild-type mice at diestrus (A), proestrus (B), estrus (C), and metestrus (D) stages. n = 3 (A–D). Scale bar = 50 μm (A–D).
Supplemental Figure S2.
Immunohistochemical staining of epithelial, stromal, and myometrial compartments in Ezh2 cKO uteri. Immunostaining of cytokeratin 8 (KRT8), vimentin (VIM), and α-smooth muscle actin (ACTA2) in 1-month–old control (Ctrl) and Ezh2 cKO uteri. n = 3 (A–F). Scale bar = 50 μm (A–F).
Supplemental Figure S3.
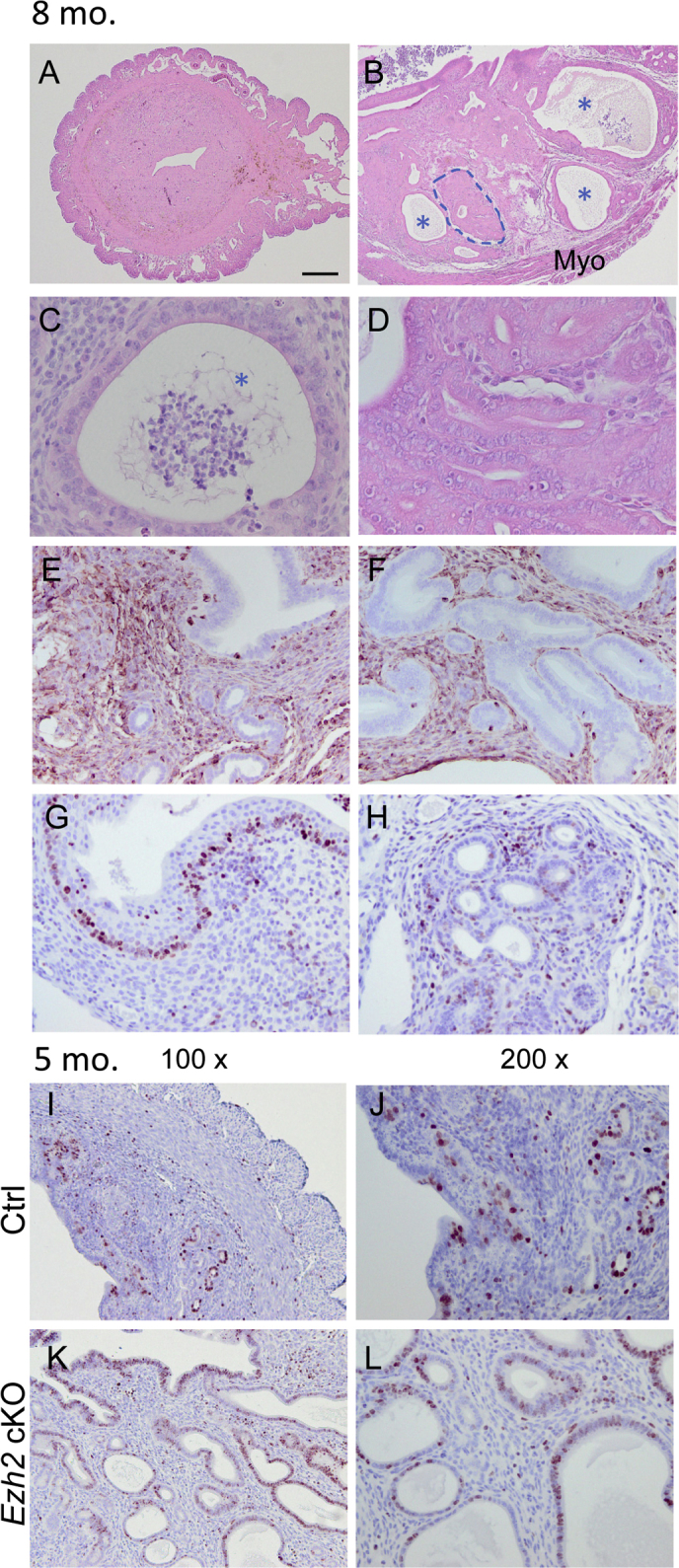
Development of endometrial hyperplasia in Ezh2 cKO uteri. A–D: Hematoxylin and eosin staining of uteri from 8-month–old control (Ctrl; A) and Ezh2 cKO (B–D) mice. B and C: Asterisks indicate cystic glands, whereas the dashed line shows an area with gland crowding, magnified in D. E and F: Vimentin staining in the uteri from control (E) and Ezh2 cKO (F) mice at the age of 8 months. G and H: Ki-67 staining in some abnormal glandular structures in the uteri of Ezh2 cKO mice at the age of 8 months. I–L: Ki-67 staining of control and Ezh2 cKO uteri at the age of 5 months. n = 3 (E–H); n = 2 to 3 (I–L). Scale bars: 250 μm (A and B); 25 μm (C and D); 50 μm (E–H, J, and L); 100 μm (I and K). Original magnification: ×100 (I and K); ×200 (J and L). Myo, myometrium.
Supplemental Figure S4.
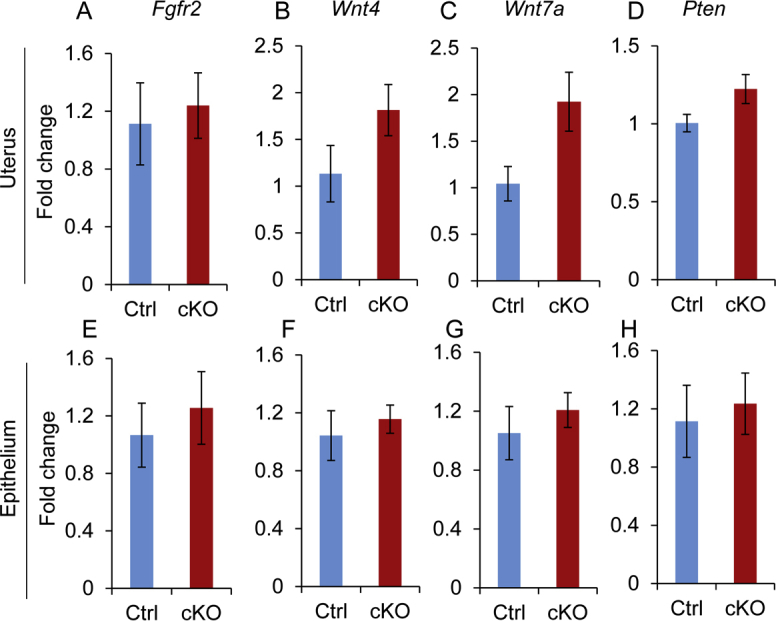
Expression of select genes associated with stratified uterine epithelia is not altered in Ezh2 cKO mice at the age of 1 month. Real-time PCR analysis of Fgfr2, Wnt4, Wnt7a, and Pten mRNA expression in uterine tissues (A–D) and uterine epithelia (E–H) isolated from control (Ctrl) and Ezh2 cKO mice. Data are expressed as means ± SEM. n = 4 uterine tissues in Ctrl mice (A–D) and uterine epithelia in Ctrl and cKO mice (E–H); n = 5 uterine tissues in cKO mice (A–D).
Supplemental Figure S5.
Elevated expression of basal cell markers in uterine epithelia isolated from Ezh2 cKO mice. A–E: Real-time PCR analysis of expression of TAp63, ΔNp63, Krt5, Krt14, and Krt17 mRNA in uterine epithelia isolated from control (Ctrl) and Ezh2 cKO mice at the age of 1 month. Data are expressed as means ± SEM (A–E). n = 4 (A–E). ∗P < 0.05, ∗∗P < 0.01.
Supplemental Figure S6.
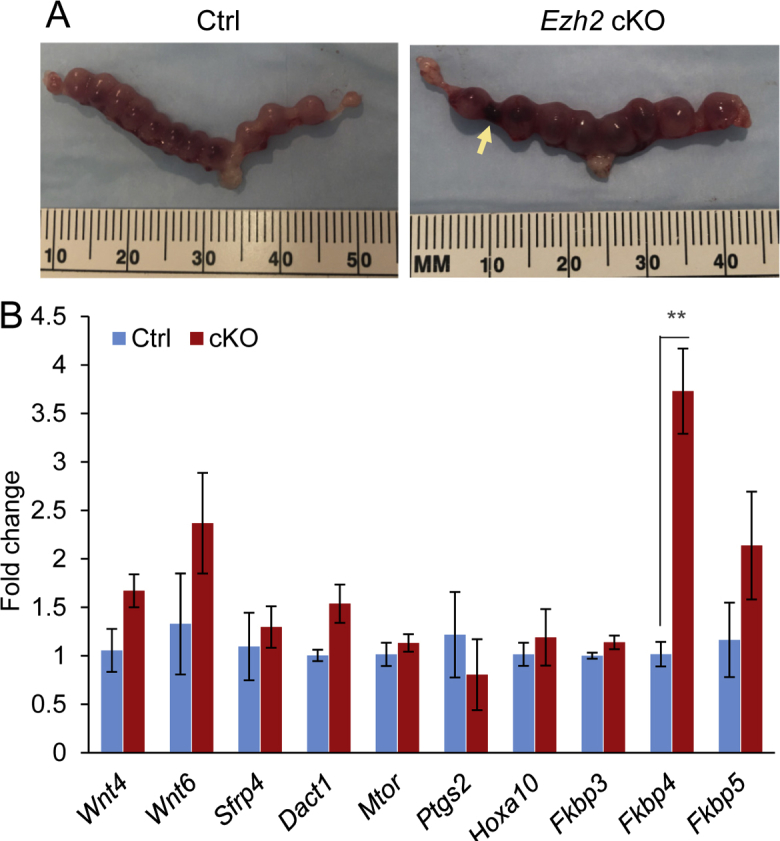
Expression analysis of genes associated with decidual differentiation and function in embryonic day 8.5 (E8.5) decidual tissues. A: Gross images of uteri from control (Ctrl) and Ezh2 cKO mice at E8.5. The arrow indicates a hemorrhagic site. B: Real-time PCR analysis of genes involved in decidualization using E8.5 decidual tissues from control and Ezh2 cKO mice. Data are expressed as means ± SEM (B). n = 3 Ctrl mice (B); n = 4 cKO mice (B). ∗∗P < 0.01.
References
- 1.Li Q., Kannan A., DeMayo F.J., Lydon J.P., Cooke P.S., Yamagishi H., Srivastava D., Bagchi M.K., Bagchi I.C. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331:912–916. doi: 10.1126/science.1197454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao Y., Li S., Li Q. Uterine epithelial cell proliferation and endometrial hyperplasia: evidence from a mouse model. Mol Hum Reprod. 2014;20:776–786. doi: 10.1093/molehr/gau033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filant J., DeMayo F.J., Pru J.K., Lydon J.P., Spencer T.E. Fibroblast growth factor receptor two (FGFR2) regulates uterine epithelial integrity and fertility in mice. Biol Reprod. 2014;90:7. doi: 10.1095/biolreprod.113.114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spencer T.E. Biological roles of uterine glands in pregnancy. Semin Reprod Med. 2014;32:346–357. doi: 10.1055/s-0034-1376354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filant J., Spencer T.E. Endometrial glands are essential for blastocyst implantation and decidualization in the mouse uterus. Biol Reprod. 2013;88:93. doi: 10.1095/biolreprod.113.107631. [DOI] [PubMed] [Google Scholar]
- 6.Kelleher A.M., Burns G.W., Behura S., Wu G., Spencer T.E. Uterine glands impact uterine receptivity, luminal fluid homeostasis and blastocyst implantation. Sci Rep. 2016;6:38078. doi: 10.1038/srep38078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray C.A., Taylor K.M., Ramsey W.S., Hill J.R., Bazer F.W., Bartol F.F., Spencer T.E. Endometrial glands are required for preimplantation conceptus elongation and survival. Biol Reprod. 2001;64:1608–1613. doi: 10.1095/biolreprod64.6.1608. [DOI] [PubMed] [Google Scholar]
- 8.Kelleher A.M., Peng W., Pru J.K., Pru C.A., DeMayo F.J., Spencer T.E. Forkhead box a2 (FOXA2) is essential for uterine function and fertility. Proc Natl Acad Sci U S A. 2017;114:E1018–E1026. doi: 10.1073/pnas.1618433114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz Y.B., Pirrotta V. Polycomb silencing mechanisms and the management of genomic programmes. Nat Rev Genet. 2007;8:9–22. doi: 10.1038/nrg1981. [DOI] [PubMed] [Google Scholar]
- 10.Sauvageau M., Sauvageau G. Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell. 2010;7:299–313. doi: 10.1016/j.stem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vire E., Brenner C., Deplus R., Blanchon L., Fraga M., Didelot C., Morey L., Van Eynde A., Bernard D., Vanderwinden J.M., Bollen M., Esteller M., Di Croce L., de Launoit Y., Fuks F. The polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 12.Wei Y., Chen Y.H., Li L.Y., Lang J., Yeh S.P., Shi B., Yang C.C., Yang J.Y., Lin C.Y., Lai C.C., Hung M.C. CDK1-dependent phosphorylation of EZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells. Nat Cell Biol. 2011;13:87–94. doi: 10.1038/ncb2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao R., Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev. 2004;14:155–164. doi: 10.1016/j.gde.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Beguelin W., Rivas M.A., Fernandez M.T.C., Teater M., Purwada A., Redmond D., Shen H., Challman M.F., Elemento O., Singh A., Melnick A.M. EZH2 enables germinal centre formation through epigenetic silencing of CDKN1A and an Rb-E2F1 feedback loop. Nat Commun. 2017;8:877. doi: 10.1038/s41467-017-01029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Q.W., Nair S., Laknaur A., Ismail N., Diamond M.P., Al-Hendy A. The polycomb group protein EZH2 impairs DNA damage repair gene expression in human uterine fibroids. Biol Reprod. 2016;94:69. doi: 10.1095/biolreprod.115.134924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimaldi G., Christian M., Steel J.H., Henriet P., Poutanen M., Brosens J.J. Down-regulation of the histone methyltransferase EZH2 contributes to the epigenetic programming of decidualizing human endometrial stromal cells. Mol Endocrinol. 2011;25:1892–1903. doi: 10.1210/me.2011-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greathouse K.L., Bredfeldt T., Everitt J.I., Lin K., Berry T., Kannan K., Mittelstadt M.L., Ho S.M., Walker C.L. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Mol Cancer Res. 2012;10:546–557. doi: 10.1158/1541-7786.MCR-11-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colon-Caraballo M., Monteiro J.B., Flores I. H3K27me3 is an epigenetic mark of relevance in endometriosis. Reprod Sci. 2015;22:1134–1142. doi: 10.1177/1933719115578924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oki S., Sone K., Oda K., Hamamoto R., Ikemura M., Maeda D., Takeuchi M., Tanikawa M., Mori-Uchino M., Nagasaka K., Miyasaka A., Kashiyama T., Ikeda Y., Arimoto T., Kuramoto H., Wada-Hiraike O., Kawana K., Fukayama M., Osuga Y., Fujii T. Oncogenic histone methyltransferase EZH2: a novel prognostic marker with therapeutic potential in endometrial cancer. Oncotarget. 2017;8:40402–40411. doi: 10.18632/oncotarget.16316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Committee for the Update of the Guide for the Care and Use of Laboratory Animals . National Academies Press; Washington, DC: 2011. National Research Council: Guide for the Care and Use of Laboratory Animals: Eighth Edition. [Google Scholar]
- 21.Soyal S.M., Mukherjee A., Lee K.Y., Li J., Li H.G., DeMayo F.J., Lydon J.P. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41:58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- 22.Shen X., Liu Y., Hsu Y.J., Fujiwara Y., Kim J., Mao X., Yuan G.C., Orkin S.H. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol Cell. 2008;32:491–502. doi: 10.1016/j.molcel.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bigsby R.M., Cooke P.S., Cunha G.R. A simple efficient method for separating murine uterine epithelial and mesenchymal cells. Am J Physiol. 1986;251:E630–E636. doi: 10.1152/ajpendo.1986.251.5.E630. [DOI] [PubMed] [Google Scholar]
- 24.Gao Y., Lin P., Lydon J.P., Li Q. Conditional abrogation of transforming growth factor-beta receptor 1 in PTEN-inactivated endometrium promotes endometrial cancer progression in mice. J Pathol. 2017;243:89–99. doi: 10.1002/path.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagashima T., Li Q., Clementi C., Lydon J.P., Demayo F.J., Matzuk M.M. BMPR2 is required for postimplantation uterine function and pregnancy maintenance. J Clin Invest. 2013;123:2539–2550. doi: 10.1172/JCI65710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang X., Ni N., Gao Y., Vincent D.F., Bartholin L., Li Q. A novel mouse model of testicular granulosa cell tumors. Mol Hum Reprod. 2018;24:343–356. doi: 10.1093/molehr/gay023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q., Agno J.E., Edson M.A., Nagaraja A.K., Nagashima T., Matzuk M.M. Transforming growth factor beta receptor type 1 is essential for female reproductive tract integrity and function. PLoS Genet. 2011;7:e1002320. doi: 10.1371/journal.pgen.1002320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paffaro V.A., Jr., Bizinotto M.C., Joazeiro P.P., Yamada A.T. Subset classification of mouse uterine natural killer cells by DBA lectin reactivity. Placenta. 2003;24:479–488. doi: 10.1053/plac.2002.0919. [DOI] [PubMed] [Google Scholar]
- 29.Gao Y., Bayless K.J., Li Q. TGFBR1 is required for mouse myometrial development. Mol Endocrinol. 2014;28:380–394. doi: 10.1210/me.2013-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo C., Balsara Z.R., Hill W.G., Li X. Stage- and subunit-specific functions of polycomb repressive complex 2 in bladder urothelial formation and regeneration. Development. 2017;144:400–408. doi: 10.1242/dev.143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu J., Lu Y., Qiao L., Ran D., Li N., Cao H., Gao Y., Zheng Q. Mouse p63 variants and chondrogenesis. Int J Clin Exp Pathol. 2013;6:2872–2879. [PMC free article] [PubMed] [Google Scholar]
- 32.Reardon S.N., King M.L., MacLean J.A., Mann J.L., DeMayo F.J., Lydon J.P., Hayashi K. Cdh1 is essential for endometrial differentiation, gland development, and adult function in the mouse uterus. Biol Reprod. 2012;86:141. doi: 10.1095/biolreprod.112.098871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ni N., Gao Y., Fang X., Melgar M., Vincent D.F., Lydon J.P., Bartholin L., Li Q.L. Glandular defects in the mouse uterus with sustained activation of TGF-beta signaling is associated with altered differentiation of endometrial stromal cells and formation of stromal compartment. PLoS One. 2018;13:e0209417. doi: 10.1371/journal.pone.0209417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guenzl P.M., Raim R., Kral J., Brunner J., Sahin E., Schabbauer G. Insulin hypersensitivity induced by hepatic PTEN gene ablation protects from murine endotoxemia. PLoS One. 2013;8:e67013. doi: 10.1371/journal.pone.0067013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao Y., Duran S., Lydon J.P., DeMayo F.J., Burghardt R.C., Bayless K.J., Bartholin L., Li Q. Constitutive activation of transforming growth factor beta receptor 1 in the mouse uterus impairs uterine morphology and function. Biol Reprod. 2015;92:34. doi: 10.1095/biolreprod.114.125146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cawthorn W.P., Bree A.J., Yao Y., Du B.W., Hemati N., Martinez-Santibanez G., MacDougald O.A. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a beta-catenin-dependent mechanism. Bone. 2012;50:477–489. doi: 10.1016/j.bone.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori H., Prestwich T.C., Reid M.A., Longo K.A., Gerin I., Cawthorn W.P., Susulic V.S., Krishnan V., Greenfield A., Macdougald O.A. Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. J Clin Invest. 2012;122:2405–2416. doi: 10.1172/JCI63604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisher D.A., Kivimae S., Hoshino J., Suriben R., Martin P.M., Baxter N., Cheyette B.N. Three Dact gene family members are expressed during embryonic development and in the adult brains of mice. Dev Dyn. 2006;235:2620–2630. doi: 10.1002/dvdy.20917. [DOI] [PubMed] [Google Scholar]
- 39.Gao Y., Wen H., Wang C., Li Q. SMAD7 antagonizes key TGFbeta superfamily signaling in mouse granulosa cells in vitro. Reproduction. 2013;146:1–11. doi: 10.1530/REP-13-0093. [DOI] [PubMed] [Google Scholar]
- 40.Spandidos A., Wang X.W., Wang H.J., Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010;38:D792–D799. doi: 10.1093/nar/gkp1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song G.A., Kim H.J., Woo K.M., Baek J.H., Kim G.S., Choi J.Y., Ryoo H.M. Molecular consequences of the ACVR1(R206H) mutation of fibrodysplasia ossificans progressiva. J Biol Chem. 2010;285:22542–22553. doi: 10.1074/jbc.M109.094557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segers I., Adriaenssens T., Wathlet S., Smitz J. Gene expression differences induced by equimolar low doses of LH or hCG in combination with FSH in cultured mouse antral follicles. J Endocrinol. 2012;215:269–280. doi: 10.1530/JOE-12-0150. [DOI] [PubMed] [Google Scholar]
- 43.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 44.Gan L., Xu M., Hua R., Tan C., Zhang J., Gong Y., Wu Z., Weng W., Sheng W., Guo W. The polycomb group protein EZH2 induces epithelial-mesenchymal transition and pluripotent phenotype of gastric cancer cells by binding to PTEN promoter. J Hematol Oncol. 2018;11:9. doi: 10.1186/s13045-017-0547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bragulla H.H., Homberger D.G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J Anat. 2009;214:516–559. doi: 10.1111/j.1469-7580.2009.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silverberg S.G. Problems in the differential diagnosis of endometrial hyperplasia and carcinoma. Mod Pathol. 2000;13:309–327. doi: 10.1038/modpathol.3880053. [DOI] [PubMed] [Google Scholar]
- 47.Sanderson P.A., Critchley H.O., Williams A.R., Arends M.J., Saunders P.T. New concepts for an old problem: the diagnosis of endometrial hyperplasia. Hum Reprod Update. 2017;23:232–254. doi: 10.1093/humupd/dmw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franco H.L., Dai D., Lee K.Y., Rubel C.A., Roop D., Boerboom D., Jeong J.W., Lydon J.P., Bagchi I.C., Bagchi M.K., DeMayo F.J. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25:1176–1187. doi: 10.1096/fj.10-175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller C., Sassoon D.A. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development. 1998;125:3201–3211. doi: 10.1242/dev.125.16.3201. [DOI] [PubMed] [Google Scholar]
- 50.Liang X.H., Daikoku T., Terakawa J., Ogawa Y., Joshi A.R., Ellenson L.H., Sun X.F., Dey S.K. The uterine epithelial loss of Pten is inefficient to induce endometrial cancer with intact stromal Pten. PLoS Genet. 2018;14:e1007630. doi: 10.1371/journal.pgen.1007630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang P., Wu S.P., Brooks K.E., Kelleher A.M., Milano-Foster J.J., DeMayo F.J., Spencer T.E. Generation of mouse for conditional expression of forkhead box A2. Endocrinology. 2018;159:1897–1909. doi: 10.1210/en.2018-00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Filant J., Lydon J.P., Spencer T.E. Integrated chromatin immunoprecipitation sequencing and microarray analysis identifies FOXA2 target genes in the glands of the mouse uterus. FASEB J. 2014;28:230–243. doi: 10.1096/fj.13-237446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee K.Y., Jeong J.W., Wang J., Ma L., Martin J.F., Tsai S.Y., Lydon J.P., DeMayo F.J. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27:5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramathal C.Y., Bagchi I.C., Taylor R.N., Bagchi M.K. Endometrial decidualization: of mice and men. Semin Reprod Med. 2010;28:17–26. doi: 10.1055/s-0029-1242989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benson G.V., Lim H., Paria B.C., Satokata I., Dey S.K., Maas R.L. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- 56.Matsumoto H., Sato E. Uterine angiogenesis during implantation and decidualization in mice. Reprod Med Biol. 2006;5:81–86. doi: 10.1111/j.1447-0578.2006.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gellersen B., Brosens I.A., Brosens J.J. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25:445–453. doi: 10.1055/s-2007-991042. [DOI] [PubMed] [Google Scholar]
- 58.Cui Y., Wang W., Dong N., Lou J., Srinivasan D.K., Cheng W., Huang X., Liu M., Fang C., Peng J., Chen S., Wu S., Liu Z., Dong L., Zhou Y., Wu Q. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012;484:246–250. doi: 10.1038/nature10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng J., Monsivais D., You R., Zhong H., Pangas S.A., Matzuk M.M. Uterine activin receptor-like kinase 5 is crucial for blastocyst implantation and placental development. Proc Natl Acad Sci U S A. 2015;112:E5098–E5107. doi: 10.1073/pnas.1514498112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamaguchi H., Hung M.C. Regulation and role of EZH2 in cancer. Cancer Res Treat. 2014;46:209–222. doi: 10.4143/crt.2014.46.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu Y., Zhang J., Guan H. Expression of EZH2 in endometrial carcinoma and its effects on proliferation and invasion of endometrial carcinoma cells. Oncol Lett. 2017;14:7191–7196. doi: 10.3892/ol.2017.7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Q., Dong P., Liu X., Sakuragi N., Guo S.W. Enhancer of zeste homolog 2 (EZH2) induces epithelial-mesenchymal transition in endometriosis. Sci Rep. 2017;7:6804. doi: 10.1038/s41598-017-06920-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ding Y.B., Long C.L., Liu X.Q., Chen X.M., Guo L.R., Xia Y.Y., He J.L., Wang Y.X. 5-Aza-2′-deoxycytidine leads to reduced embryo implantation and reduced expression of DNA methyltransferases and essential endometrial genes. PLoS One. 2012;7:e45364. doi: 10.1371/journal.pone.0045364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O'Carroll D., Erhardt S., Pagani M., Barton S.C., Surani M.A., Jenuwein T. The polycomb-group gene Ezh2 is required for early mouse development. Mol Cell Biol. 2001;21:4330–4336. doi: 10.1128/MCB.21.13.4330-4336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dunlap K.A., Filant J., Hayashi K., Rucker E.B., 3rd, Song G., Deng J.M., Behringer R.R., DeMayo F.J., Lydon J., Jeong J.W., Spencer T.E. Postnatal deletion of Wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol Reprod. 2011;85:386–396. doi: 10.1095/biolreprod.111.091769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jeong J.W., Lee H.S., Franco H.L., Broaddus R.R., Taketo M.M., Tsai S.Y., Lydon J.P., DeMayo F.J. beta-Catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene. 2009;28:31–40. doi: 10.1038/onc.2008.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Snitow M.E., Li S., Morley M.P., Rathi K., Lu M.M., Kadzik R.S., Stewart K.M., Morrisey E.E. Ezh2 represses the basal cell lineage during lung endoderm development. Development. 2015;142:108–117. doi: 10.1242/dev.116947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galvis L.A., Holik A.Z., Short K.M., Pasquet J., Lun A.T., Blewitt M.E., Smyth I.M., Ritchie M.E., Asselin-Labat M.L. Repression of Igf1 expression by Ezh2 prevents basal cell differentiation in the developing lung. Development. 2015;142:1458–1469. doi: 10.1242/dev.122077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mills A.A., Zheng B.H., Wang X.J., Vogel H., Roop D.R., Bradley A. p63 Is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 70.Yalcin-Ozuysal O., Fiche M., Guitierrez M., Wagner K.U., Raffoul W., Brisken C. Antagonistic roles of Notch and p63 in controlling mammary epithelial cell fates. Cell Death Differ. 2010;17:1600–1612. doi: 10.1038/cdd.2010.37. [DOI] [PubMed] [Google Scholar]
- 71.Murray-Zmijewski F., Lane D.P., Bourdon J.C. p53/p63/p73 Isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ. 2006;13:962–972. doi: 10.1038/sj.cdd.4401914. [DOI] [PubMed] [Google Scholar]
- 72.Koster M.I., Kim S., Mills A.A., DeMayo F.J., Roop D.R. p63 Is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004;18:126–131. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Candi E., Rufini A., Terrinoni A., Dinsdale D., Ranalli M., Paradisi A., De Laurenzi V., Spagnoli L.G., Catani M.V., Ramadan S., Knight R.A., Melino G. Differential roles of p63 isoforms in epidermal development: selective genetic complementation in p63 null mice. Cell Death Differ. 2006;13:1037–1047. doi: 10.1038/sj.cdd.4401926. [DOI] [PubMed] [Google Scholar]
- 74.Laurikkala J., Mikkola M.L., James M., Tummers M., Mills A.A., Thesleff I. p63 Regulates multiple signalling pathways required for ectodermal organogenesis and differentiation. Development. 2006;133:1553–1563. doi: 10.1242/dev.02325. [DOI] [PubMed] [Google Scholar]
- 75.Romano R.A., Ortt K., Birkaya B., Smalley K., Sinha S. An active role of the DeltaN isoform of p63 in regulating basal keratin genes K5 and K14 and directing epidermal cell fate. PLoS One. 2009;4:e5623. doi: 10.1371/journal.pone.0005623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Margueron R., Li G.H., Sarma K., Blais A., Zavadil J., Woodcock C.L., Dyniacht B.D., Reinberg D. Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol Cell. 2008;32:503–518. doi: 10.1016/j.molcel.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ezhkova E., Lien W.H., Stokes N., Pasolli H.A., Silva J.M., Fuchs E. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 2011;25:485–498. doi: 10.1101/gad.2019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ezhkova E., Pasolli H.A., Parker J.S., Stokes N., Su I.H., Hannon G., Tarakhovsky A., Fuchs E. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell. 2009;136:1122–1135. doi: 10.1016/j.cell.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]