Abstract
Traumatic brain injury (TBI) remains one of the most prevalent forms of morbidity among Veterans and Service Members, particularly for those engaged in the conflicts in Iraq and Afghanistan. Neuroimaging has been considered a potentially useful diagnostic and prognostic tool across the spectrum of TBI generally, but may have particular importance in military populations where the diagnosis of mild TBI is particularly challenging, given the frequent lack of documentation on the nature of the injuries and mixed etiologies, and highly comorbid with other disorders such as posttraumatic stress disorder, depression, and substance misuse. Imaging has also been employed in attempts to understand better the potential late effects of trauma and to evaluate the effects of promising therapeutic interventions. This review surveys the use of structural and functional neuroimaging techniques utilized in military studies published to date, including the utilization of quantitative fluid attenuated inversion recovery (FLAIR), susceptibility weighted imaging (SWI), volumetric analysis, diffusion tensor imaging (DTI), magnetization transfer imaging (MTI), positron emission tomography (PET), magnetoencephalography (MEG), task-based and resting state functional MRI (fMRI), arterial spin labeling (ASL), and magnetic resonance spectroscopy (MRS). The importance of quality assurance testing in current and future research is also highlighted. Current challenges and limitations of each technique are outlined, and future directions are discussed.
Keywords: Traumatic brain injury, Magnetic resonance imaging, Diffusion tensor imaging, fMRI, Positron emission tomography, Magnetic resonance spectroscopy, Veteran
Traumatic brain injury (TBI) is one of the more prevalent injuries in the military,affecting readiness, unit effectiveness, and the general safety of self and others (see Helmick et al. 2015). The historical role of neuroimaging forTBI both in military and civilian settings has generally relied upon computed tomography (CT)for determining injury severity based upon visualization of the distribution and nature of lesions, for detecting operable lesions requiring immediate surgical intervention, and for monitoring lesion change over time post-trauma and/or post-operatively. However, advanced imaging techniques have garnered significant interest in recent years, and while not yet utilized in clinical settings, these new modalities are being evaluated and developed for use in the clinical management and treatment planning beyond their current role as research applications.
Imaging holds promise as a useful tool in a number of applications in military populations. First, imaging has been offered as a potential “biomarker” for the diagnosis of mild TBI (mTBI), which currently relies on subjective assessments and self-reports ofsymptoms, particularly in military populations where objective information on initial injury severity is often not recorded or is inaccessible in medical records. Second, imaging has been used to evaluate the interplay between TBI and commonly associated comorbidities such as posttraumatic stress disorder (PTSD), depression, and substance misuse, with the assumption that these disorders may have dissociable patterns on imaging or that they may have cumulative effects in areas affected by each of these disorders. Third, there has been significant interest in differences that may exist between blast-related mTBI as compared to other injury mechanisms involving direct impact to the head. Fourth, imaging could be used in examining long-term changes associated with exposure to injury, particularly neurodegenerative conditions such as chronic traumatic encephalopathy (CTE) (Koerte et al. 2015b). Fifth, imaging may enhance understanding of dose–response relationships in individuals exposed to multiple impacts or blasts. Sixth and more recently, imaging has held promise in the evaluation of potential rehabilitation strategies and pharmacologic interventions for Service Members and Veterans with TBI. Seventh, though controversial, advanced imaging may eventually play a role in the consideration of existing vulnerabilities that may render an individual more prone to the effects of mTBI, and as such, may potentially assist in the determination of compensation and benefits.
The majority of the published reports involving advanced neuroimaging in Veterans and Service Members with TBI focus upon mTBI sustained during the conflicts in Iraq and Afghanistan (Operation Iraqi Freedom [OIF], Operation Enduring Freedom [OEF], and Operation New Dawn [OND]). This likely reflects the increased survival on the battlefield as well as increasing prevalence of combat-related mTBI. Increased research funding directed toward understanding and alleviating the consequences of mTBI over the past decade may also be a factor, but the coincident evolution of imaging acquisition and analysis methods that enable widespread use of advanced imaging techniques is also contributory. In this review of imaging findings among Veterans and Service Members with TBI, the major advanced imaging methods are described, and a general overview of current findings in the military and Veteran population is provided. Though there are limited reports of the use of some of these techniques in populations of Veterans and Service Members at the present time, they are reviewed as potential avenues of future research. Challenges and limitations of each modality are discussed, and future applications of each technique are also suggested. We also direct the reader to the other papers in this special issue, which provide additional novel imaging-related results not contained within this review as well as more detailed discussions of the specific types of injuries most commonly sustained in Service Members and the complexities of comorbidities in this population.
Lesion and volumetric imaging
Technique
Conventional structural magnetic resonance imaging (MRI) includes a number of sequences routinely used in both clinical and research settings. MRI uses the combination of safe high strength magnetic field (as opposed to ionizing radiation as in computed tomography - CT), controllable magnetic field gradients, and radiofrequency (RF) pulses to produce different image types that are uniquely sensitive to various pathological changes that occur after a head injury. Sequences discussed here include the T1-weighted (T1-w) anatomical sequence, T2-weighted (T2-w), fluid attenuated inversion recovery (FLAIR) sequences, and T2* gradient recalled echo (GRE) or susceptibility weighted images (SWI). The T1-w sequence is a conventional method used primarily to create high-resolution structural images of the brain that result in contrast between basic tissue types, namely white/gray matter and cerebrospinal fluid (CSF). This makes T1-w imaging useful for evaluation of a number of abnormalities including lesions, gray and white matter volumetric changes, and cortical thickness. T2-w and FLAIR sequences extend the sensitivity of T1-w imaging to include detection of macroscopic white matter lesions, contusions, and intra-cerebral bleeding, changes in cerebrospinal fluid composition and ventricular volume, and changes in epidural and/or subdural volumes and/or contents. Thus, the foremost clinical application of T1-w, T2-w, and FLAIR images in the context of TBI is the identification of pathology produced by trauma (diffuse axonal injury and frank lesions) and the tracking of its progression over time. Acquired in concert, these scans are far more sensitive for the detection of brain abnormalities than is CT imaging alone (Gandy et al. 1984). SWI is a high-resolution gradient echo (GRE) scan that is sensitive to subtle changes in the susceptibility (i.e., density and/or composition) of the underlying tissue. This allows this sequence to visualize venous blood, microhemorrhages, hemosiderin, calcifications and/or other changes to vasculature (e.g., deep medullary veins) typically not observed in more traditional structural sequences. Importantly, this sequence is growing in popularity and usefulness, especially in mTBI.
Current uses in military/veteran TBI studies
Lesion and volumetric studies have been conducted in civilian populations since theinception of MRI. Lesion studies in civilian populations demonstrate the heterogeneity of lesion location and a relationship between lesion number and volume with severity of injury. In addition, volumetric studies in the civilian literature generally show atrophy of both gray and white matter structures with increasing severity (for a review of civilian studies see Shenton et al. 2012). The vulnerability of the frontal and temporal lobes is especially well-demonstrated with this modality (Wilde et al. 2005; Reider et al. 2002; Bigler et al. 2002), including a trajectory of atrophy thatmay continue for some time after injury (Farbota et al. 2012; Bendlin et al. 2008).
In contrast, there are a limited number of studies in more recent military and Veteran populations (OEF/OIF) that focus exclusively on lesions or volumetric analyses. Some studies will report the existence of lesions in studies of Veterans (Levin et al. 2010; Raymont et al. 2010), though lesions are clearly not the focus of these studies. Only a single study examining traditional volumetric differences in Veterans with TBI is published in the recent literature. More specifically, a study by Lopez-Larson and colleagues (2013) demonstrated bilateral volumetric enlargement of the thalamic nuclei in Veterans with TBI who were positive for overt suicidal behaviors, while no significant enlargement was noted in the thalami of Veterans with a history of TBI who did not also have overt suicidal behaviors (Lopez-Larson et al. 2013). Volumetric enlargement was thus interpreted as being associated with suicidal behavior.
In addition to more traditional volumetric studies (region of interest or structure of interest), cortical thickness is commonly measured using the T1-w sequence. Two studies (one in active duty Service Members and one in Veterans) demonstrated significant cortical thinning in a number of areas. The first study by Tate et al. found significant thinning in the Heschl’s gyrus (left hemisphere only) among a small sample of Service Members exposed to primary blast injury (Tate et al. 2014). The sample was noted to have a significant amount of hearing/auditory clinical abnormalities including tinnitus, ruptured tympanic membrane, hearing loss, and other auditory abnormalities. For this reason, thinning was interpreted as being related to hearing/auditory abnormalities rather than more direct effects of blast injury on the brain. The second study by Corbo et al. (2014) reported that Veterans with especially long lifetime histories of PTSD symptoms displayed cortical thinning across a number of cortical areas. Importantly, the Veterans with comorbid PTSD and mTBl showed greater and more diffuse thinning associated with elevated measures of stress (Corbo et al. 2014). These findings suggest that mTBI may exacerbate the vulnerability of the brain to stressful events and situations, though it is clear that additional studies are needed in this population.
To date, there do not appear to be studies examining white matter hyperintensities (WMH) or other T2-w/FLAIR abnormalities in military and/or Veteran cohorts. Anecdotally, WMH are commonly observed in Service Member and/or Veteran participants with mTBI, and examination of these findings may yield additional information as studies in civilian TBI populations have demonstrated modest relationships between WMH volume and clinical outcomes including TBI severity (Bigler et al. 2013), functional outcomes (Marquez de la Plata et al. 2007), and atrophic changes (Ding et al. 2008).
Currently, there also appear to be no studies specifically examining SWI abnormalities in military or Veteran populations. However, SWI is excellent at detecting subtle lesions, and it is known to be more sensitive in detecting pathological abnormalities when compared to T2-weighted or FLAIR sequences (Tong et al. 2003). Civilian studies have shown an increased number and volume of SWI lesions in more severe TBI patients (Haacke et al. 2013). Importantly, these studies also demonstrate a number of important relationships between increases in SWI lesions and worse functional outcomes including length of hospital stay, functional outcome measures, and intellectual function (Beauchamp et al. 2013; Ashwal et al. 2006).
Anecdotally, there are a significant number of military and Veteran TBI patients with SWI abnormalities, even those classified as mTBI. Given the significant functional relationships noted in previous civilian clinical studies, this sequence should be utilized in military and Veteran populations as a means of further characterizing injury severity.
Limitations, challenges and future directions
Clearly, one of the current limitations is the small number of recent publications in military and Veteran populations using lesion and volumetric studies. This will likely be remedied over the next few years as there are a significant number of investigators who now have access to a growing number of military and Veteran participants. In fact, this special issue illustrates this point. However, instead of simply applying older methods and technology to these data, a careful examination of the methods and results from the civilian literature could improve time to discovery in the Veteran population and lead to important findings of clinical significance, especially those that improve our ability to predict functional outcomes.
Another limitation of current volumetric studies is the general lack of methods that can detect changes in the individual patient who have experienced a TBI. Heterogeneity of injury location is well known, though much of the literature continues to examine differences between groups of patients or subjects. This has often lead to a number of seemingly contradicting results across the literature as well as reinforcing the belief that mTBI may not represent an authentic form of brain injury. Going forward, methods that emphasize analyses aimed at characterizing the volumetric changes in the individual patient and the functional relevance of these changes will be best suited to improve our understanding of TBI brain and functional sequelae (see comparisons of individual mTBI civilian subjects with persistent symptoms by Bouix et al. 2013 for example).
The majority of the TBI cases incurred in military and Veteran populations fall within the mild range (http://dvbic.dcoe.mil/sites/default/files/DoD-TBI-Worldwide-Totals-2014-Q1-Q4-Feb23–2015.pdf; Helmick et al. 2015), resulting in more subtle changes in cellular microstructure. These changes are far more difficult to observe and measure using conventional structural imaging methods. However, methods specifically designed to examine these subtle changes in gross anatomy, including shape analyses, might reveal more specific biomarkers of brain injury. The changes in volume or shape of an area of injured brain also is likely related to time since injury and dependent on underlying subcortical and white matter alterations. As a result, structural imaging methods should not be examined in isolation and should be combined with other more sensitive sequences including diffusion tensor, metabolic, and functional imaging to improve sensitivity and specificity. Finally, although the majority of individuals with mTBI do not demonstrate evidence of overt lesions observed using conventional imaging, there are certainly cases of mTBI that, while the individual does not manifest more than “mild” injury on initial physical examination, do reveal frank lesions, the size and location of which may be important in relation to outcome and in interpreting other forms of imaging (see Fig. 1).
Fig. 1.
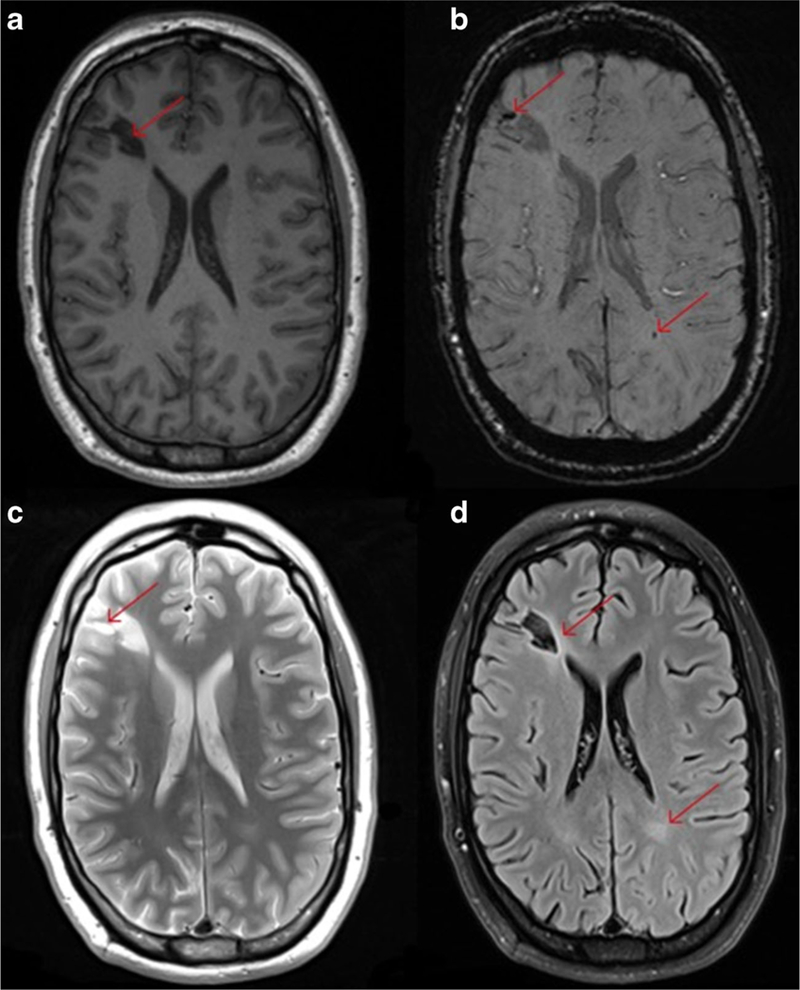
This figure illustrates the different contrast available when using structural MRI to examine a young OIF/OIF Active Duty Service Member diagnosed with a mild TBI (LOC<30 min; AOC<24 h; PTA <24 h). Utilization of multimodal imaging (even structural imaging) can be informative with each sequence potentially adding additional clinically meaningful information about the specific injury incurred. Panel a shows the T1-weighted image and the large hypointense lesion in the right frontal lobe (red arrow). Panel b shows the SWI image and not only shows the large hypointense lesion but several smaller hemosiderin deposits including one in the white matter in the right parietal occipital region (red arrows). Panel c is the T2-weighted image and shows the bright areas indicating inflammation or CSF accumulation around the larger lesion. Panel d is the FLAIR image and shows enhancement around the larger lesion and an area abnormality in the white matter in the left parietal occipital region (contracoup injury)
Future studies that include SWI as a prominent sequence in the analysis could lead to additional refinement of TBI severity classification algorithms, especially in the military/Veteran population where current criteria exclude from the mTBI category any patient who has positive clinical imaging findings (e.g., day of injury CT). Inclusion of additional improved analysis will reduce the amount of manual, and more labor intensive methods. Newer, more automated methods for examining volumetric and lesion data are constantly under development, and utilization of these newer methods may prove to be important in further characterizing TBI abnormalities.
Diffusion tensor imaging
Technique
Diffusion tensor imaging (DTI) is an MR imaging technique that measures the diffusion of water in tissue and models this diffusion process in each voxel as a three-dimensional ellipsoid. The mathematical representation of this ellipsoid is a rank-2 symmetrical tensor which quantifies the size, shape and orientation of the diffusion (Basser and Pierpaoli 1996). Several features have been derived from the tensor to describe these properties, and we describe below the measures most commonly used in neuroimaging research:
Fractional anisotropy (FA) is a scalar between 0 and 1 describing the shape of the tensor. Zero corresponds to an isotropic diffusion (the ellipsoid is a sphere) such as in unrestricted free water, and 1 is an extreme anisotropic diffusion (the ellipsoid reduces to a single line). Anisotropic diffusion results when the motion of water molecules is restricted in a particular direction. For example, in white matter, axonal membranes, filaments, and/or myelin sheaths, will restrict water to diffuse slower across the fibers than along the fibers. In the normal human brain, FA is highest in white matter (WM), lower in gray matter (GM) and lowest in cerebrospinal fluid (CSF). Figure 2 represents different hypothetical tensor shapes that may be associated with different FA values. Note that the lowest FA would be rendered as a perfect sphere, where water molecules are free to move equally in any direction. In contrast, the highest FA values would be reflected by tensor shapes that are cylindrical, where the motion of water molecules preferentially follow one direction.
Mean diffusivity (MD) quantifies the size of the tensor, i.e., the average amount of diffusion in a voxel.
Axial diffusivity (AD) is the length of the long axis of the ellipsoid (the largest eigenvalue of the tensor).
Radial Diffusivity (RD) is the average length of the middle and short axes of the ellipsoid (the middle and smallest eigenvalues of the tensor averaged).
Fig. 2.
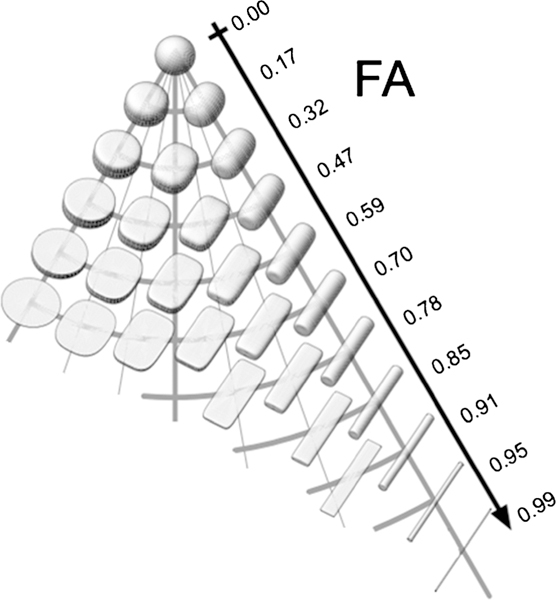
Visualization of the Fractional Anisotropy of Diffusion Tensors. Note that tensors of different shapes can have the same FA. Adapted from Ennis and Kindlmann (Ennis and Kindlmann 2006)
In addition, tractography, a post-processing technique which reconstructs major fiber pathways based on the orientation of the tensor, can provide insight into the architecture of the white matter (Ito et al. 2002). Figure 3 below demonstrates tensor orientation and applied tractography using DTI. DTI is particularly sensitive in evaluating WM microstructure and has become an increasingly popular imaging technique in TBI research, in particular mTBI, where diffuse axonal injury is prevalent but findings on conventional imaging may be unrevealing (Shenton et al. 2012; Aoki et al. 2012).
Fig. 3.
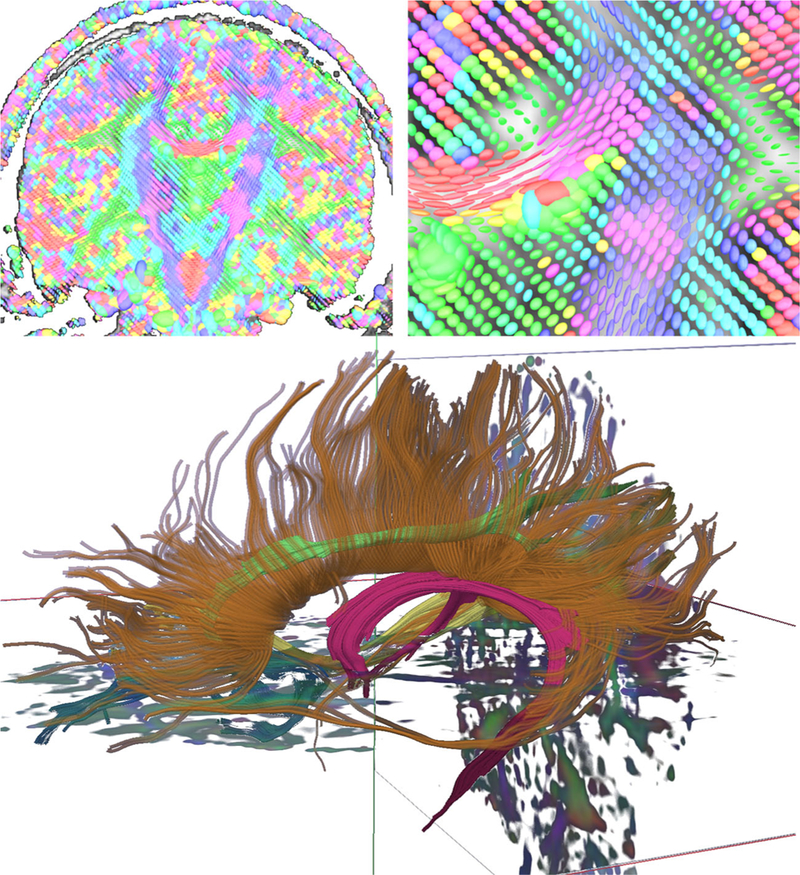
Top Left: A coronal slice of a DTI volume at the level of the posterior internal capsule. The image is composed of many individual tensor estimations, and the orientation of these estimations indicates the presumed direction of the fiber. Consistent with convention, red indicates fibers coursing in a right-left orientation, green represents an anterior-posterior fiber orientation, and blue/purple reflects a superior/inferior orientation. Top right: Magnification of an area near the junction of the corpus callosum and internal capsule showing highly organized anisotropic tensors in the corpus callosum. Bottom: Tractography of the corpus callosum (brown), fornix (magenta), and cingulum bundle (green)
Current uses in military/veteran TBI studies
Although there are several studies utilizing DTI in mTBI in civilians, studies utilizing diffusion imaging in Veterans and Service Members with mTBI are more limited. Mac Donald et al. (2011) examined diffusion-related abnormalities occurring in the early stages of mTBI in deployed service personnel who had been evacuated from the field due to injury. DTI-derived parameters of 63 Service Members who had experienced mTBI were compared with those of 21 Service Members with no history of TBI, and the authors found that Service Members with mTBI demonstrated lower FA in the cerebellar peduncles, cingulate bundles, and orbitofrontal white matter relative to the comparison group. These changes appeared to be persistent in a subgroup (N=47) of these Service Members who underwent follow-up imaging 6–12 months later (Mac Donald et al. 2011). In a subsequent study of a small group (n=4) of OEF/OIF service personnel with a single reported primary blast-related mTBI that were imaged 2–4 years after exposure, reduced FA was also found in the left middle cerebellar peduncle and left superior cerebellar peduncle of these individuals as compared to a comparison group of returning military personnel from Iraq and Afghanistan without history of head injury (Mac Donald et al. 2013). These studies utilized region of interest analysis approaches, as well as an additional automated template-based segmentation approach in the latter study.
However, others have not detected group differences between Service Members and Veterans with and without a reported history of TBI using voxel-based analyses (Davenport et al. 2012; Jorge et al. 2012) or tractography (Levin et al. 2010). Levin et al.’s initial study comparing 37 OIF/OEF Veterans and Service Members with chronic phase mild to moderate blast-related TBI and 15 Veterans without a history of TBI or exposure to blast revealed no differences on DTI-based measures of diffusion (FA and apparent diffusion coefficient or ADC), though DTI measures were related to cognitive performance in some domains (Levin et al. 2010). Jorge et al. used DTI to examine white matter integrity in a relatively larger group (N=72) of Iraq and Afghanistan Veterans with a history of mTBI as compared to a comparison group of deployed Veterans without a history of TBI (N=21) (Jorge et al. 2012). While traditional voxel-based analyses did not reveal group differences, application of a method of identifying spatially heterogeneous areas of decreased FA (called “potholes”) did suggest that Veterans with mTBI had a significantly higher number of potholes than those without history of TBI.
Davenport et al. (2012) also addressed the issue of potential spatial heterogeneity of white matter abnormalities across individuals by analyzing the distribution of FA values across total white matter voxels in OIF/OEF Veterans with and without a history of mTBI (Davenport et al. 2012). In this study, the number of voxels with low FA (i.e., two or more standard deviations from the control group mean) was greater in the group of Veterans exposed to blasts. Finally, Taber et al. (2015) found lower FA and higher RD in Veterans exposed to primary blast with (n=6) and without (n=23) mTBI relative to blast-unexposed (n =16) Veterans. Moreover, voxel clusters of lower FA were spatially dispersed and heterogeneous across affected individuals (Taber et al. 2015).
Morey et al. (2013) compared OEF/OIF Veterans with mTBI and (n=30) comorbid PTSD and depression (n=30) to primary (n=42) and confirmatory (n=28) comparison groups with no history of TBI using high angular resolution diffusion imaging (HARDI) which allows for whole brain voxelwise analyses of multiple crossing fibers (Morey et al. 2013). The diffusion measure utilized, partial volume fraction, of the primary fiber in a voxel was found to be lower in the TBI group in diffuse cortical and subcortical tracts including the body of the corpus callosum, genu of the corpus callosum, splenium of the corpus callosum, forceps minor, forceps major, superior corona radiata, posterior corona radiata, posterior limb of internal capsule, posterior thalamic radiation, retrolenticular part of internal capsule, superior longitudinal fasciculus, and the tapetum. Furthermore, several of these findings were related to conventional measures of injury severity including loss of consciousness (LOC) and to the presence of feeling dazed and confused (alteration of consciousness (AOC)).
When considering the effects of comorbid PTSD (common in military and Veteran samples) Davenport et al. (2015) demonstrated that PTSD was consistently associated with high generalized FA (a generalization of the FA measure for high angular resolution diffusion imaging data) in select brain regions, greater likelihood of regions and voxels with abnormally low MD, and a greater number of voxels with abnormally high FA, while mTBI was associated with fewer high MD regions (Davenport et al. 2015). In a study examining the interplay between TBI and PTSD in OEF/OIF Veterans, Bazarian et al. concluded that PTSD severity was related to both the severity of combat stress and underlying structural brain changes using DTI but not to a clinical diagnosis of mild TBI (Bazarian et al. 2013). Others have not found PTSD to have a significant effect on DTI parameters in the context of TBI (Morey et al. 2013). The effect of mood disorders, such as depression, may also augment changes observed in Veterans with mTBI and/or PTSD (Isaac et al. 2015; Matthews et al. 2011b) and DTI-based differences have also been linked to suicidal behavior in the context of mTBI in Veterans (Lopez-Larson et al. 2013; Yurgelun-Todd et al. 2011). Finally, the impact of alcohol misuse (with or without comorbid PTSD) has been the subject of study (Maksimovskiy et al. 2014), and is another important comorbidity in OEF/OIF Veterans. See Table 1 for a review of subject characteristics, imaging parameters and basic findings.
Table 1.
DTI studies in military mild TBI (in chronological order)
| Author | TBI participant characteristics and comparison |
Military cohort | Time post-injury | Field strength and acquisition details |
Analysis method(s) | Summary of findings |
|---|---|---|---|---|---|---|
| Levin et al. 2010 |
Patients: 37 TBI (mild to moderate) Age: 31.5±7.2 years Controls: 15 controls Age: 31.4±5.4 year (Gender not specified) |
OIF/OEF Veterans | Chronic 871.5±343. 1 days post-injury | 3T Philips Achieva; Voxel size=2.0×2.0×2.0 mm FOV=224×224 mm TR/TE=7341/70 ms; 32 diffusion directions; b-value=1000 s/mm2 |
Quantitative tractography; single slice region of interest, voxel-based analysis Variables: FA, ADC |
DTI using tractography, standard single-slice region-of-interest measurement, and voxel-based analysis disclosed no groupdifferences in FA or ADC. However, FA of the left and right posterior internal capsule and left corticospinal tract was positively correlated with total words consistently recalled, whereas ADC for the left and right uncinate fasciculi and left posterior internal capsule was negatively correlated with this measure of verbal memory. Correlations of DTI variables with symptom measures were non-significant. |
| Mac Donald et al. 2011 |
Patients: 63 mTBI (63M, OF) Age: 24 (19–58) years Controls: 21 controls (21M, OF) Age: 31 (19–49) years |
Active Duty Service Members (evacuated from field) | Subacute Median time from injury to enrollment= 14 days (range, 1 to 90) Follow-up 62–12 months post-injury |
Two 1.5 T Siemens MAGNETOM Avanto; voxel size=2.5 × 2.5 × 2.5 mm; FOV = not stated TR/TE= 10200/102 ms 23 diffusion directions b-value = not stated | Multi-slice region of interest using Analyze v6.1 Variables: RA, AD, RD, MD |
Service Members with mTBI demonstrated lower FA in the cerebellar peduncles, cingulum bundles, and orbitofrontal white matter relative to the comparison group. These changes appeared to be persistent in a subgroup (N=47) of these Service Members who underwent follow-up imaging 6–12 months later. |
| Matthews et al. 2011b |
Patients: 11 mTBI w/ MDD (11M, OF) Age: 26.8 (22–45) years Controls: 11 mTBI w/o MDD (11M, OF) Age: 30.3 (22–47) years |
OIF/OEF Veterans | Chronic mTBI w/ MDD: 2.8±1.0 years after most severe blast-related concussion mTBI w/o MDD: 3.3±1.1 years after most severe blast-related concussion | 3T GE Signa EXCITE; 3 mm slice thickness; FOV=240 mm × 240 mm; TR= 10900 ms 61 difiusion directions; b-value=1500 s/mm2 | Conventional voxel-based analysis Variables: FA |
Veterans with MDD evidenced lower FA in the bilateral corona radiata, left superior corona radiata, anterior corpus callosum, and left superior longitudinal fasciculus than Veterans without MDD.In the MDD group, lower FA in the left superior longitudinal fasciculus correlated with greater depressive symptoms. |
| Yurgelun-Todd et al. 2011 |
Patients: 15 mild-moderate/severe TBI (15M, OF) Age: 34.9±9.7 years Controls: 17 healthy controls (17M, OF) Age: 34.0±10.6 years |
Patients: Veterans Controls: Veterans and Civilians |
Unspecified | 3T Siemens MAGNETOM Trio; voxel size=2.0×2.0×2.0 mm; FOV=256×256 mm; TR/TE=9000/88 ms; 64 difiusion directions; b-value=1000 s/mm2 | FSL’s Difiusion Toolbox; White matter tracts created using JHU-White Matter Label Atlas Variables: FA, MD |
Veterans with TBI exhibited lower FA than healthy controls in the left cingulum bundle, left genu of the corpus callosum, and total genu of the corpus callosum.No group differences in MD were found for any white matter tracts. In the TBI group, FA of the right and total cingulum bundle positively correlated with suicidal ideation at the time of screening. FA in the left cingulum, total cingulum, and right genu were positively correlated with higher impulsivity. |
| Jorge et al. 2012 |
Patients: 72 mTBI (72M, OF) related to blast exposure during deployment Age for “possible TBI”: 29.6±6.9 years Age for “probable TBI”:28.8±10.6 years Controls: 21 controls (21M, OF) Age: 33. 7±5.4 years |
Veterans | Chronic Possible TBI: 51.8±19.2 months Probable TBI: 47.0±20.5 months |
3T Siemens TIM Trio; Slice thickness=2.0 mm FOV=256×256 mm, TR/TE=8700/82 ms; 64 difiusion directions; b-value=1750 s/mm2 | Conventional voxel-based analysis as well as a method of identifying spatially heterogeneous areas of decreased FA called “pothole” analysis Variables: FA, MD |
Voxel-based analysis did not reveal differences in DTI parameters between the veterans with mild TBI and those with no TBI. However, the Veterans with mild TBI had a significantly higher number of potholes than those without TBI. The difference in the number of potholes was not influenced by age, time since trauma, a history of mild TBI unrelated to deployment, or coexisting psychopathology. The number of potholes was correlated with the severity of TBI and with performance in executive functioning tasks. |
| Davenport et al. 2012 |
Patients: 25 mTBI (24M, IF) Age: 36.0±8.9 years Controls: 33 controls (28M, 5F) Age: 32.5±8.6 years |
OIF/OEF Military Service Members | Chronic 2–5 years post-injury | 3T Siemens TIM Trio; Slice thickness=2.0 mm; FOV=256×256 mmTR/TE=9000/84 ms; 30 diffusion directions; b-value=800 s/mm2 | Region of interest analysis using 20 standard probabilistic tractography-based ROIs, as well as comparison of the number of ROIs with “low” average FA without requiring the same regions to be abnormal across participants and comparison of voxels with FA in the lower end of the healthy distribution, not necessarily those with FA statistically lower than the healthy mean. Variables FA, MD | Blast mTBI was associated with a diffuse, global pattern of lower white matter integrity, and this pattern was not affected by previous civilian mTBI. Neither type of mTBI had an effect on the measures sensitive to more concentrated and spatially consistent white matter disruptions. Additionally, individuals with more than one blast mTBI tended to have a larger number of low FA voxels than individuals with a single blast injury |
| Mac Donald et al. 2013 |
Patients: 4 mTBI 4 mTBI with “primary blast” (3M, IF) Age: 30 (23–36) years Controls: 18 controls (18M, OF) Age: 31 (19–49) years |
Service Members from OIF/OEF | Chronic 2–4 years post-injury | 1.5 T Siemens Magnetom Avanto; voxel size=2.5 × 2.5 × 2.5 mm; FOV=not stated TR/TE= 10200/102 ms 23 diffusion directions b-value=not stated | Region of interest analysis, as well as an additional automated template-based segmentation approach (DTIStudio) Variables: RA, AD, RD, MD |
Reduced FA found in the left middle cerebellar peduncle and left superior cerebellar peduncle of these individuals as compared to a comparison group of returning military personnel from Iraq and Afghanistan without history of head injury. |
| Morey et al. 2013 |
Patient: 30 mTBI (29M, IF) Age: 39.6+10.8 years Controls: 42 non-lBI primary group (32M, 1 OF) Age: 36.8±9.9 years 28 non-TBI confirmatory group matched with TBI group on alcohol and drug use, gender, and education (26M, 2F)Age: 37.5+11.3 years |
Service Members and Veterans from OIF/OEF | Chronic 9.7±10.8 years post-injury | GE 3T EXCITE; voxel size=2.0×2.0×2.0 mm; FOV=240×240 mm; TR/TE=17000/76 ms; 55 diffusion directions; b-value=1000 s/mm2 | TBSS; BEDPOSTX performed crossing fiber analysis that analyzed the anisotropy of each voxel that can be attributed to primary fibers (f1) and the anisotropy that can be attributed to crossing fibers (f2). Variables: FA, f1, f2 |
f1 was significantly lower in the TBI group in diffuse white matter regions. Lower f1 predicted longer LOC in diffuse white matter regions. FA analyses showed a similar association but had different voxels of significance from f1 in the brain stem. There were no significant results for f2. |
| Bazarian et al. 2013 |
Patients: 30 subjects identified in clinical interview as “almost certainly” or “very likely” to have sustained a TBI during deployment Controls: 22 subjects “not at all likely” to “somewhat likely” to have sustained a TBI All: 49M,3F Age: 30.8±7.1 years |
OIF/OEF Veterans | Chronic Approximately 4 years after last tour | 3T Siemens Trio; FOV= 256×256 mm; TR/TE= 8900/81 ms; 60 diffusion directions; b-value=700 s/mm2 | Custom software in MATLAB, C++, and FSL processed and registered the images; FAST Toolbox in FSL performed whole brain analysis; JHU White Matter Parcellation Atlas perform the ROI segmentation Variables: FA, MD | FA and MD of various white matter tracts associated with PTSD, blast exposure, and TBI. PTSD severity associated with higher first percentile values of MD. Blast exposure associated with lower first percentile values of FA. |
| Lopez-Larson et al. 2013 |
Patients: 40 TBI - SB (40M,0F) Age: 34.6±8.1 years 19 TBI + SB (19M,0F) Age: 38.0±7.8 years Controls: 15 controls (15M,0F) Age: 36.5± 11.5 years |
Veterans | Unspecified | 3T Siemens Trio; Voxel size=2.0×2.0×2.0 mm, FOV=256×256 mm; TR/TE=9000/88 ms; 64 diffusion directions; b-value= 1000 s/mm2 | FSL’s FMRIB’s Diffusion Toolbox; JHU-White Matter Label Atlas Variables: FA | Differences in thalamic volumes and AIR fractional anisotropy (FA) were examined between (1) TBI + SB versus HC and (2) TBI + SB versus combined HC and TBI-SB and (3) between TBI + SB and TBI-SB. Left and right thalamic volumes were significantly increased in those with TBI + SB compared to the HC, TBI-SB, and the combined group. Veterans with TBI + SB had increased FA bilaterally compared to the HC,HC and TBI-SB group, and the TBI-SB only group. Significant positive associations were found for bilateral AIR and BIS in the TBI + SB group. |
| Taber et al. 2015 |
Patients: 6 Blast-exposed mTBI (6M,0F) Age: 35.8±8.7 years 23Blast-exposed (18M,5F) Age: 35.8±7.4 years Controls 16 controls (12M,4F) Age: 37.3 ±11.5 years |
Veterans (deployed after 2001) | Chronic Greater than 1 year post-injury | 1.5 T General Electric Signa HDxt; Voxel size=2.0×2.0×6.0 mm; FOV=240× 192-mm; TR/TE=8,500 ms/107 ms; 25 diffusion directions; b=1000 s/mm2 | TBSS; also quantification of the number of voxel clusters with altered DTI metrics, regardless of their spatial location Variables: FA, AD and RD | Significantly lower FA and higher RD was observed in veterans exposed to primary blast with and without mild TBI relative to blast unexposed veterans. Voxel clusters of lower FA were spatially dispersed and heterogeneous across affected individuals. |
| Davenport et al. 2015 |
patients: 19 mTBI w/o PTSD (18M,1F) Age: 36.6±9.4 years 31 PTSD w/o mTBI (29M,2F) Age: 33.3 ±9.3 years 45 mTBI + PTSD (45M,0F) Age: 30.2±6.2 years Controls: 38 controls (32M,6F) Age:33.9±8.8 years |
OIF/OEF Veterans | Chronic 2–5 years post-injury | 3T Siemens Trio; thickness=2.0 mm FOV=256×256 mm TR/TE=9,000/84 ms 30 diffusion directions, b-value=800 s/mm2 | FSL Voxelwise Comparison Variables: FA, MD, and GFA | PTSD was consistently associated with high GFA in select brain regions, greater likelihood of regions and voxels with abnormally low MD, and a greater number of voxels with abnormally high FA, while mTBI was associated with fewer high MD regions.Overall, PTSD was associated with more restricted diffusion (low MD) and greater anisotropy (high GFA) in regions of crossing/diverging fibers poorly characterized by a single tensor (FA), suggesting that interstitial fibers may be involved. Contrary to earlier results in a sample without PTSD, mTBI was not associated with anisotropyabnormalities, perhaps indicating the co-occurrence of PTSD and mTBI requires special consideration with regard to structural brain connectivity. |
| Isaac et al. 2015 |
Patients: 25 mTBI, PTSD, and Depression (23M,2F) Age: 46.0±4.6 years 20 mTBI and PTSD w/o Depression (18M,2F) Age: 44.0±3.3 years No Control Group |
Veterans | Chronic | 3T GE Discovery MR750; Voxel size=2.5×2.5×2.5 mm; FOV=250×250 mm; TR/TE=6600/80 ms; 30 diffusion directions; b-value = 1000 mm/s2 | mrDiffusion freeware performed alignment and registration and whole brain tractography; Custom MATLAB programming identified fiber tracts using JHU-White Matter Label Atlas Variables: FA, AD, RD | DTI-based predictive statistical model correctly classified 84 % of patients with TBI, PTSD, and depression but only correctly classified 12 % of patients with PTSD and TBI but not depression. FA Left uncinate fasciculus and right cingulum bundle were predictors of the group to which the subject belonged. RD and AD were not significant predictors of group classification. |
Abbreviations: FA fractional anisotropy, RA relative anisotropy, AD axial diifusivity, RD radial diifusivity, MD mean diifusivity, GFA generalized FA, MDD major depressive disorder, JHXJ Johns Hopkins University, HC healthy control, SB suicidal behavior, OIF/OEF Operation Iraqi Freedom/Operation Enduring Freedom, FOV field of view, TR/TE repetition time/echo time, FSL FMRIB Software Library, TBSS tract-based spatial statistics, LOC loss of consciousness, ATR anterior thalamic radiations, BIS Barratt Impulsiveness Scale
Limitations, challenges and future directions
Currently, there are a number of disparate findings in the literature when using DTI to examine the effects of mTBI in military and Veteran populations that complicate our understanding. Importantly, there may be a number of methodological differences that explain these equivocal findings including differences in the diffusion acquisition parameters, imaging artifact, field strength, and analysis tools used. In addition, cohort factors including the definition of TBI, the mechanism of injury (primary blast versus blast injury in addition to another mechanism, such as head impact), inclusion/exclusion criteria for the subject groups (including consideration regarding injuries sustained prior to or after the index injury), and different comparison populations (deployed military vs. non-deployed military, VA-based recruitment vs community-based Veterans, Veterans vs. Active Duty Service Members, etc.), length of post-injury interval, and control for common comorbidities such as PTSD and depression, etc. could have also contributed to different findings.
Unfortunately, studying DTI abnormalities in TBI subjects remains additionally challenging, because the impact of the injury is very heterogeneous, and is unlikely to affect the brains of different subjects in exactly the same way. The methods used in most studies rely on standard population analyses, which assume a common spatial pattern of pathologies over the entire patient group. As such, they have limited sensitivity and utility. This is reflected in recent reviews of dMRI findings in mTBI that highlight the disparity in findings (Shenton et al. 2012). In addition, recent results in animal models and humans suggest that DTI indices in GM might reveal important information about mTBI, but most DTI analysis tools available today concentrate only on the analysis of the white matter (Budde et al. 2011; Bouix et al. 2013). Recent techniques have tried to address this problem by creating full brain subject-specific profiles of injury based on a reference atlas built from healthy subjects (Bouix et al. 2013; Kim et al. 2013; Mayer et al. 2014; Jorge et al. 2012; Davenport et al. 2015). This issue is even more interesting in military population with blast exposure where one could expect both subject specific abnormalities and a common pattern of injury from exposure to blast.
Another limitation of DTI is its non-specificity. While DTI measures are very good at detecting an abnormality, they do not necessarily provide detailed information related to the specific underlying pathology associated with that abnormality. For example, lower FA in WM could be caused by a number of pathologies, such as myelin deficiency, axonal death, edema, etc. Earlier work suggested that DTI metrics may reflect specific forms of pathology, for example, increased radial diffusivity being associated with demyelination (Song et al. 2005), and altered axial diffusivity shown to be sensitive markers for axonal damage (Song et al. 2002, 2003; Wang et al. 2014, 2015a). More specific features can be extracted from DTI, although they still need to be validated (Pasternak et al. 2009). Alternatively, one could acquire more advanced diffusion MRI scans, such as multi-shell diffusion imaging, diffusion spectral imaging or neurite orientation dispersion and density imaging, which provide more specific markers of brain tissue microstructure than standard DTI indices (Descoteaux et al. 2011; Wedeen et al. 2008; Zhang et al. 2012; Wang et al. 2015a; Wang et al. 2014). Unfortunately, they often require long scan times and extensive postprocessing expertise, rendering their clinical application difficult.
One exciting area of research is the use of animal models to correlate better the diffusion MRI signal with physiological, metabolic, and pathological consequences of blast TBI. One interesting result from an animal model of blunt trauma is the correlation of FA and histology. In particular, increased FA in GM was correlated with increased glial markers and decreased FA in WM was correlated with decreased myelin markers (Budde et al. 2011). Unfortunately, few if any of the existing studies in this domain have employed advanced diffusion imaging beyond DTI (Tompkins et al. 2013; Calabrese et al. 2014; Budde et al. 2013; Rubovitch et al. 2011). Future research endeavors in this domain have the potential to provide fundamental insight into the neurotrauma caused by TBI biomechanics, especially blast forces.
Magnetization transfer imaging
Technique
Magnetization transfer imaging (MTI) is a form of imaging that examines the interaction (dipolar and/or chemical exchange) between “free” water protons and “bound” macromolecular protons to assess the presence or absence of macromolecules, particularly the proteins and phospholipids that coat axonal membranes and myelin sheaths in cerebral white matter. MTI is achieved via measurement of magnetization interaction when an off resonance radio frequency (RF) pulse is applied to macromolecular protons and some of their excited magnetization is transferred to free water protons. Through application of this pulse, the resulting signal is attenuated according to the magnitude of magnetization transfer between the macromolecules initially pulsed and the bulk water. The presence or absence of macromolecules can be inferred depending on the degree of attenuation of the signal.
MT ratio (MTR) has historically been the most commonly applied quantitative MTI parameter, and is calculated as the percent variation of the saturated RF pulse image sequence with the unsaturated RF pulse image sequence (Pagani et al. 2008). The equation is as follows,
where M0 is the sequence without the RF pulse and Ms is the signal intensity with the RF pulse. This represents the percent signal decrease due to MT.
MTR has proven to be a sensitive as a clinical marker of pathologic change in numerous neurological diseases and generally, decreased MTR is thought to reflect pathological change in both white and gray matter. MTR has been previously reported to be useful in probing myelin integrity, and also to detect inflammation and associated edema in other disease processes (Wang et al. 2015b; Harrison et al. 2014). It has been postulated that decreased MTR in TBI may reflect an increase in the concentration of microglia, amyloid, phagocytic vacuoles and/or other injury products within both the gray and white matter. MTI is thought to be particularly sensitive to white matter demyelination and Wallerian degeneration because the myelin sheath surrounding axons holds a substantial number of tethered proteins that transfer magnetization (Symms et al. 2004).
Current uses in military/veteran TBI studies
There are a limited number of MTI studies in TBI, even in the civilian literature, but decreased MTR in white matter regions has been reported in the absence of any observable pathology on conventional imaging (Sinson et al. 2001; Mamere et al. 2009), including in patients with mTBI (McGowan et al. 2000), although a recent study did not find significant differences between a group of individuals with subacute mTBI and an orthopedically-injured comparison group imaged within 24–48 h post-injury (Narayana et al. 2015). In military TBI specifically, Petrie and colleagues (2014) utilized macromolecular proton fraction (MPF), based on magnetization transfer effect, in a population of Iraq and Afghanistan Veterans with blast-related mTBI. They found reduced MPF in multiple cortical-subcortical tracts in groups of individuals with blast-related TBI compared to controls and that MPF reduction was related to the degree of blast exposure (Petrie et al. 2014).
Limitations, challenges and future directions
As with all imaging modalities, there are special considerations related to the acquisition, analysis and interpretation of MTI data (Hunter et al. 2012). MTI sequences can be lengthy, increasing their vulnerability to motion artifact, which can influence MTR values substantially. Second, quantitative results obtained with different acquisition parameters or scanner hardware or software may not be directly comparable across sites (Pagani et al. 2008). Third, although MTR may be considered sensitive to alteration in the microstructual environment, its ability to reveal specific forms of underlying pathology (e.g., inflammation, edema, Wallerian degeneration, demyelination) requires further investigation, particularly in TBI where multiple forms of pathology may co-occur. MTI-based measures may also be quite sensitive to age-related changes, particularly in cortical and subcortical GM structures (Mascalchi et al. 2014; Newbould et al. 2014; Callaghan et al. 2014). Finally not all studies have found a clear association between MTI-derived metrics and outcome (Bagley et al. 2000; Petrie et al. 2014), and additional research in this area is warranted.
Despite these challenges, MTI techniques show promise in detecting subtle white and gray matter changes in otherwise normal-appearing tissue following TBI and could advance understanding of transient or persistent microstructural alteration following mTBI. Development of quantitative MT measures including the concentration of macromolecular pool, free pool size ratio (PSR) (Dortch et al. 2013), and exchange rates between pools may reflect additional information related to underlying pathological processes. Newer methods for MTI that do not require a separate saturation pulse are under development, which may yield larger MTR, higher SNR and greater gray-white matter contrast in a shorter amount of time (e.g., ~1–2min) (Barker etal. 2015). Additionally, alternatives to spoiled gradient echo (GRE) approaches to MT imaging such as balanced steady state free precession (bSSFP) have also been proposed to reduce scan time considerably (Amann et al. 2015). There has also been limited, but intriguing investigation of the use of MTI in monitoring neurological recovery (Choe et al. 2013), axonal “remodeling” after injury (Lin et al. 2014) and the effects of pharmacological intervention (Zivadinov et al. 2014) in other patient populations.
Arterial spin labeling
Technique
Arterial spin labeling (ASL) is a non-invasive MRI technique that uses magnetically-labeled blood water as an endogenous contrast agent to provide measurements of cerebral blood flow (CBF). Without the use of a gadolinium-based contrast agent, quantitative maps of CBF, in terms of perfusion per unit of tissue (mL/100 g/min) can be obtained when certain acquisition conditions are met (Detre et al. 1992). Clinically, this technique can be used to investigate pathologies that can increase or decrease CBF, such as ischemia (Yoo et al. 2015; Hartkamp et al. 2014), disruption of the blood brain barrier, and cerebrovascular spasm (Cernak and Noble-Haeusslein 2010; Ling et al. 2009; Bauman et al. 2009). In addition, ASL has been applied to populations with more overt or subtle forms of vascular disease and neurodegeneration.
In ASL, the longitudinal magnetization in the blood, typically flowing from the carotid and vertebral arteries, is saturated or inverted, resulting in the reduction of the T1 -weighted signal. This is called the “tagged” image. The difference between these tagged images and images with no tagging (controls) is proportional to the CBF as long as the labeled blood arrives to the tissue when the tissue is imaged. Since the difference between the two images is very small, multiple tagged and control images are acquired to increase the signal-to-noise ratio (SNR) of these measurements. Several types of ASL acquisition sequences exist on modern scanners. A recent consensus white paper on ASL concluded that pseudocontinuous ASL (pCASL) is the preferred choice for clinical ASL given its high tagging efficiency by providing temporally longer labeled bolus at lower RF energy depositions (Alsop et al. 2014).
Current uses in military/veteran TBI studies
Although the principle of ASL has been utilized in research applications for several years, the recent improvements of MR hardware and sequence development have now made ASL more widely available on modern scanners. Therefore, in contrast to other MR techniques like DTI and fMRI, there is a lack of studies published particularly in military-related TBI. In civilian studies of mTBI, researchers have shown alterations in CBF both in the acute and chronic phase. Doshi et al. (2015) showed an increase in CBF in a group of 14 individuals with acute mTBI, which is in contrast to the decrease in CBF typically seen in severe TBI. Using quantitative SWI to measure the oxygen demand of the tissue, they showed that there may be a neuroprotective mechanism of increased CBF above the needed oxygen demand (Doshi et al. 2015). In pediatric patients with TBI in the chronic phase, Wang et al. (2015b) demonstrated decreased CBF in bilateral frontotemporal regions (Wang et al. 2015c). ASL can also be used to look at changes in CBF due to interventions such as pharmacological interventions for addictive disorders (Franklin et al. 2012) and attention deficit (Kim et al. 2012b). This makes ASL particularly attractive for investigating effects from interventions for TBI. Additionally, as an alternative for BOLD contrast for fMRI, ASL has also been used for direct measure of CBF changes in task-based fMRI (Kim et al. 2012a).
Limitations, challenges and future directions
CBF measurements have been useful in PET studies in neurodegenerative disease, and ASL is an attractive, accessible and nonionizing alternative means to investigate these pathologies. With recent interest in TBI-related changes that may contribute to or accelerate neurodegenerative disease through different mechanisms, ASL may provide an important avenue of future research of cerebral blood flow in military-based studies. Additionally, there is interest in blast-related changes to cerebral perfusion that may be persistent and could be potentially evaluated with ASL. However, it is important to note that there may be some differences in absolute CBF measurements between the two modalities. Zhang et al. showed that pCASL had higher regional CBF in cortical areas in comparison to 15O PET CBF measurements (Zhang et al. 2014). Attention should also be given to CBF measurements in white matter. Due to the longer transit time needed for white matter, a bias can be introduced which can affect white matter measurements. The SNR in white matter regions are also generally lower. Mutsaerts et al. (2014) has also reported a difference in white matter CBF when comparing pCASL sequences between vendors (Mutsaerts et al. 2014). Several investigators are looking at ways to improve measurements in these areas such as calibrating to dynamic susceptibility contrast (DSC) perfusion to ASL (Zaharchuk et al. 2010) and updated ASL techniques with improved acquisition sequences (Wolf et al. 2014; Wang et al. 2013) and post-processing techniques (Wang 2014).
Magnetic resonance spectroscopy
Technique
Magnetic resonance spectroscopy (MRS) is a noninvasive technique that examines physiological metabolismin vivo. MRS differs from other imaging methods in TBI in that it measures the concentration of chemicals in the brain. These chemicals are often involved in metabolic processes in the brain, thus providing a window into the underlying pathological changes that can occur as a result of brain injury from acute to chronic stages (Lin et al. 2012b). This quantitative, non-invasive, and objective technique has demonstrated its value in diagnosis and prognosis, across a broad range of neurological diseases including cancer, neurodegenerative diseases, demyelinating disorders, inherited metabolic disorders, stroke, hypoxia, and others (Oz et al. 2014). Specific chemical changes identified by MRS are also amenable to targeted treatment and treatment-monitoring. Available across all MR scanners as a software option, this research technique shows great promise to be readily translated into clinical practice.
The major metabolites measured by MRS in TBI are the following (also see Fig. 4):
Fig. 4.
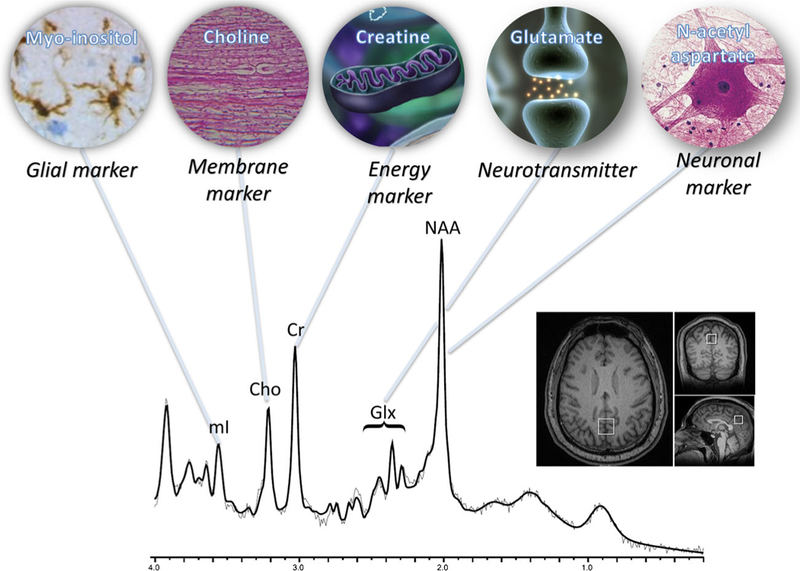
Major metabolite resonances and associated biological functions. Each peak is labeled with the abbreviation and inset which describes the role that each metabolite plays as biomarkers for traumatic brain injury. Data acquired using single voxel, PRESS, TR/TE: 2000/30 ms, 2×2×2 cc in posterior cingulate at 3Tas shown in bottom right inset and postprocessed using LCModel. Modified from Lin et al. (2012a)
Lipid:
Although lipids are present throughout the brain in the form of membranes, they are not “MR visible” unless liberated by a severe pathological process including brain trauma (Haseler et al. 1997).
Lactate:
Lactate is the end product of anaerobic glycolysis and therefore a direct indicator of hypoxic events in the brain. The presence of lactate in spectra indicates impairment of perfusion and is indicative of poor outcome (Makoroff et al. 2005).
N-acetyl aspartate (NAA):
An amino-acid derivative synthesized in neurons and transported down axons, NAA is a marker of viable neurons, axons and dendrites. Brain injury is associated with decreased NAA (Ross et al. 1998; Signoretti et al. 2001; Moffett et al. 2013).
Glutamate and glutamine (Glx):
Glutamate is the primary excitatory neurotransmitter in the brain and is tightly coupled to glutamine which is found in the astrocytes. Studies have shown that the Glx resonance is predictive of outcome after severe TBI (Shutter et al. 2004). Importantly, it should be noted that Glx is not visible at long echo.
Creatine (Cr):
Creatine is an energy marker that is often used as an internal reference for the measurement of other peaks. Earlier MRS TBI studies utilize ratios of metabolite to Cr. However, recent studies have shown changes in Cr as a result of head injury and therefore these ratios must be considered carefully (Gasparovic et al. 2009; Yeo etal. 2011).
Choline (Cho):
Choline is a membrane marker used to measure the changes in brain tissue. Since the majority of choline-containing brain constituents are not normally soluble, pathological alterations in membrane turnover result in an increase in MRS visible Cho. In the context of head injury, Cho is often elevated and described as a marker of diffuse axonal injury (Holshouser et al. 2005). Myo-inositol (ml): mI is an astrocyte marker and osmolyte. It is also involved in the metabolism of phosphatidyl inositol, a membrane phospholipid, and, similar to choline, is expected to increase after TBI due to membrane damage. Another explanation for increased mI after TBI is that mI is a purported glial marker and increases as a result of reactive astrocytosis or glial scarring (Ashwal et al. 2004). Importantly, mI is not visible at long echo.
Current uses in military/veteran TBI studies
Although there have been few studies that have utilized MRS to studymilitary TBI, the methods are highly applicable to that population. There have been several recent reviews of brain injury that have focused on the use of magnetic resonance spectroscopy from mild (Lin et al. 2012b) to severe (Marino et al 2011) brain injury as well as more specific topics including repetitive brain injury (Ng et al. 2014), sports-related head injury (Gardner et al. 2014) that provide a comprehensive list of relevant literature (see also Table 2 for a review of basic imaging parameters and findings in MRS studies in mild TBI). In general, the studies show an initial decrease in NAA after injury which then recovers over time. Choline changes appear to be more variable and as it relates to membrane turnover or diffuse axonal injury may be dependent on the type and extent of the brain injury. Changes in Glx and mI, tied to excitoxicity and glial cell proliferation, respectively, appear to be more long-standing. It is important to note that both Glx and mI are only observed using short-echo spectroscopy which is the reason why other studies, which utilized long-echo methods, did not detect these changes. Recent new findings include the use of 3D chemical shift imaging methods (Kirov et al. 2013a, 2013b) which provide greater spatial sensitivity of MRS to injury as well as 2D correlated spectroscopy methods which provide two chemical shift domains that allows for the disambiguation of overlapping resonances (Lin et al. 2015).
Table 2.
MRS studies in mild TBI (civilian and military in chronological order)
| Author | TBI participant characteristics and comparison cohort |
Time post-injury | Field strength and MRS method |
Brain region(s) | Summary of findings |
|---|---|---|---|---|---|
| Cecil et al. 1998 |
Patients: 35 mTBI (17M,18F) Age:40 (21–77) yrs GCS: 13–15 Controls: 12controls (6M, 6F) Age: 28 (21–19) yrs |
Chronic 2–3066 days post-injury |
1.5 T; STEAM SV, TE=31 ms | Splenium | • ⇩NAA/Cr in the splenium |
| Garnett et al. 2000 |
Patients: 8 mTBI Age: 41 (22–66) yrs GCS: 14–15 Controls: 8 controls |
Subacute 3–11 days post injury |
1.5 T; STEAM SV, TE=30 ms | Centrum semiovale | • ⇧Cho/Cr in the mTBI group. • ⇩NAA/Cho in the mTBI group. |
| Kirov et al. 2007 |
Patient: 20 mTBI (12M, 8F) Age: 36(19–59) yr GCS: 13–15 Controls: 17 controls (9M, 12F) Age: 38 (19–61) yrs |
Chronic 7 days – 7 years post-injury (mean 2 year) |
3T; PRESS CSI, TE= 135 ms | Thalamus | • Prospective study of the thalamus that defines the minimal level of detection between mTBI and control. |
| Cohen et al. 2007 |
Patients:20 mTBI (11M, 9F) Age: 35 (19–57) yrs GCS: 13–15 Controls: 19 controls (11M, 8F) Age: 39 (21–61) yrs |
Chronic 9 days – 9 years post-injury (mean 2 year) |
1.5 T; Whole brain NAA (Cohen et al. 2007) | Whole brain | • ⇩ in NAA when compared with controls. |
| Vagnozzi et al. 2008 |
Patients: 28 mTBI (28M) Age: 27(22–34) yrs Controls: 5 controls Age: 22–34 |
Subacute 3,15,30 days post-injury Sports-related TBI |
3T; PRESS SV, TE=144 ms | Bilateral frontal WM | • Longitudinal study showed ⇩ NAA/Cr and recovery to baseline except in those patients that suffered a second concussion in which recovery was slower and incomplete at 30 days post-injury. |
| Gasparovic et al. 2009 |
Patients: 10 mTBI (4M, 6F) Age: 29 (21–19) yrs GCS: 13–15 Controls: 9 controls (4M, 5F) Age:28 (21–49) |
Subacute 4–19 days |
3T; PRESS SVand CSI, TE=40 ms | Splenium and supraventricular GM and WM | • Both single voxel and multivoxel methods to showed same results • ⇩Glx in GM in mTBI • ⇧Cr in WM in mTBI |
| Yeo et al. 2011 |
Patients: 32 mTBI (14M, 18F) Age: 27.3±9.5 years GCS: 13–15 Controls: 32 controls (14M, 18F) Age: 26.87±9.24 |
Subacute 5–21 days |
3T; PRESS CSI, TE=40 ms |
Supraventricular GM and WM |
• Longitudinal study showed ⇧Cr and⇧Glx in WM • ⇧Glx in GM. • Recovery of the metabolites was observed. |
| Henry et al. 2011 |
Patients: 10 concussed athletes Age: 22.5 years Controls: 10 non-concussed athletes, age-matched |
Acute and Chronic 1–6 days, 6 months Sports-related TBI |
3T; PRESS SV, TE=30 ms | Motor cortex, dorso-lateral prefrontal cortex, hippocampus | • Longitudinal study found ⇩NAA/Cr in DLPFC and M1 in acute phase with some recovery in chronic phase. • ⇧ mI was found in the chronic phase. |
| Maugans et al. 2012 |
Patients: 12 children (9M, 3F) Age:11–14 Controls: 10 non-concussed athletes, age-matched |
Acute to Subacute <72 h, 14 days, 30 days post-injury Sports-related TBI |
3T; PRESS SV, TE=144 ms | Anterior cingulate gyrus, left dorsolateral prefrontal white matter, and left thalamus |
• Longitudinal study of child athletes showed no significant changes in brain metabolites of NAA or Cho. |
| Johnson et al. 2012 |
Patients: 15 student athletes (6M, 9F) Age: 20.6±1.2 Controls: 15 non-concussed athletes (7M, 8F) Age: 20.4±0.8 |
Subacute 10.8 days after injury but within 24 h of symptom resolution Sports-related TBI |
3T; 3D PRESS CSI, TE=135 ms |
genu and splenium of the corpus callosum | • No change in NAA/Cho and NAA/Cr in the genu and splenium. • Co relation with DLPFC and hippocampal functional interhemispheric connectivity |
| Chamard et al. 2013 |
Patients: 10 University level athletes (10F) Age: 22±2 Controls: 10 Healthy athletes (10F) Age: 21±1 |
Chronic =7 months post-concussion Sports-related TBI |
3T; PRESS SV, TE=30 ms | Bilateral hippocampus, DLPFC, m1 | • ⇩ mI in hippocampus and m1 bilaterally. • DTI showed no change in FA but ⇧ MD. • ⇩FA in corpus callosum fibers projecting to m1. |
| Hetherington et al. 2014 |
Patients: 25 veterans (24M) Age: 34±9 Controls: 10 Healthy contorls (12M, 13F) Age: 32±12 |
Chronic =1 year post-concussion Mild: Blast-related (Military) |
7 T; 2D CSI, TE=40 ms | Bilateral hippocampus | • ⇩ NAA/Cr bilaterally and NAA/Cho (likely ⇧ Ch0) in right hippocampus. • Novel use of shimming array to overcome technical problems in hippocampus. |
| Kirov et al. 2013a |
Patients: 26 ER mTBI patients (21M, 5F) Age: 33±11 Controls: 13(8M, 5F) Healthy controls Age: 33±12 |
Acute/Subacute 3–55 days post-injury |
3T; 3D PRESS CSI, TE=35 ms | GM, WM, corpus callosum | • ⇩ NAA in WM. • Cho and Cr positively correlate with time from mTBI. |
| Kirov et al. 2013b |
Patients: 26 ER mild TBI patients (21M, 5F) Age: 33±11 Controls: 13(8M, 5F) Healthy controls Age: 33 ±12 |
Acute/Subacute 3–55 days post-injury |
3T; 3D PRESS CSI, TE=35 ms | GM, WM, corpus callosum | • Similar to previous study except that group was broken down to PCS+ (n= 11) and PCS- (n=15). • PCS+ showed ⇩NAA in WM. • Cho and Cr did not correlate with time from mTBI in either PCS+ or PCS−. |
| Poole et al. 2015 |
Patients: 34 high-school American football athletes (all M) Age: 15–18 years Controls: 10 (all M) healthy athletes Age: 15–18 years |
Acute/Subacute Pre-season, 1, 2, and 3 months during season Subconcussive only Sports-related |
3T; PRESS SV, TE=30 ms | DLPFC, Ml | • No player concussed during season, therefore only subconcussion observed. • ⇩Cr in DLPFC during the season in Team 2 • Higher ml in DLPFC at baseline but ⇩ml over season in Team 2 • ⇩Cho in Ml |
| Lin et al. 2015 |
Patients: 5 professional athletes (4 football, 1 baseball, all M) Age: 43.6± 10.8 years Controls: 5 healthy (all M) Age: 45.2 ±12.6 years |
Chronic 3–25 years post-injury Sports-related |
3T; COSY SV, Initial TE=30 ms, 64 increments of 0.8 ms | PCG | • ⇧ Glu + Gin • ⇧ Cho • ⇧ Phe • ⇧ Fucosylated molecules • NAA and ml not different |
| Koerte et al. 2015 |
Patients: 11 former professional soccer players (all M) Age: 52.0±6.8 years Controls: 14 non-contact sports athletes (all M) Age: 46.9±7.9 |
Chronic >10 years after play Subconcussive only Sports-related |
3T; PRESS SV, TE=30 ms | PCG | • ⇧ Cho in PCG • ⇧ ml in PCG • mI and GSH correlated with lifetime estimate of repetitive head injury. |
Abbreviations: STEAM stimulated echo acquisition method; PRESS point-resolved spectroscopy; SV single voxel; CSI chemical shift imaging; COSY correlated spectroscopy; TE echo time, ms milliseconds; WM white matter, GM grey natter; DLPFC dorsal lateral prefrontal cortex, NAA n-acetyl aspartate; Cr creatine; Cho choline; Glx glutamate/glutamine; Phe phenylalanine
The results have been less consistent due to a number of different factors that influence the more subtle changes in concussions (Ng et al. 2014). Age can be a factor as most studies in adults have shown reductions in NAA after concussion (Gasparovic etal. 2009; Yeo etal. 2011; Cecil etal. 1998; Garnett et al. 2000; Cohen et al. 2007; Kirov et al. 2007; Vagnozzi et al. 2008; Henry et al. 2011; Johnson et al. 2012), whereas children have not (Maugans et al. 2012), which is surprising given non-spectroscopic evidence of worse outcome in children (Guskiewicz and Valovich McLeod 2011). Given the limitations of a single study, it is unclear if NAA metabolism is maintained in the pediatric brain or that in this specific cohort, the injuries did not affect neuronal integrity. Gender can also play a role with recent studies demonstrating differences in metabolic changes in women (Chamard et al. 2012, 2013). Variation in data acquisition methods including differences in pulse sequences and regions of interest which can be influenced by grey and white matter differences have also led to differences observed in Cho, Glx, and mI concentrations (Gardner et al. 2014). Heterogeneity of injury with regards to location as well as number of concussions also influence metabolic changes. Longitudinal MRS studies in mTBI have also shown that metabolites can recover over time and therefore the time after injury (acute vs chronic) must also be taken into account when evaluating spectral differences (Yeo et al. 2011; Vagnozzi et al. 2008; Henry et al. 2011; Chamard et al. 2012). Similar findings have also been shown for repetitive brain injury such as those suffered by sports athletes (Henry et al. 2011; Lin et al. 2010, 2015). These repetitive injuries may be of greater interest to the military, given the repeated exposures to blast injury and importance of this fact bearing on “return-to-duty” decisions. Furthermore recent studies have also shown exposure to repetitive sub-concussive injuries may also have an effect on brain chemistry (Poole et al. 2014, 2015; Koerte et al. 2015a).
To date, there has only been one study utilizing MRS to study brain injury in military personnel (Hetherington et al. 2014). Hetherington et al. examined 25 veterans at least 1 year after exposure to 1 or more (>10) blasts and compared them to age and gender matched healthy controls with no history of brain injury. This study utilized cutting-edge methods such as a 7 Tesla MRI scanner, specialized transceiver array coil and gradient insert to optimize signal to noise ratio and MRS signal homogeneity. These technological advances are necessary in order to obtain high quality spectra from regions such as the temporal lobes which often suffer from susceptibility artifacts. The results of the study showed that veterans had significantly decreased NAA/Cr and NAA/Cho ratios (possible increased Cho) similar to previously reported findings in other non-military chronic injuries. One of the interesting findings, particularly for a military cohort, was there was no significant difference between TBI subjects with and without post-traumatic stress, anxiety, depression, or alcohol dependence, which are the major co-morbidities in military TBI. This is surprising given that MRS studies in military subjects with PTSD have shown changes in hippocampus such as decreased NAA and changes in Cho (Brown et al. 2003; Freeman et al. 1998; Kimbrell et al. 2005; Schuff et al. 2008) that may have shown a compounding effect.
Limitations, challenges and future directions
Clearly there is a need for more studies examining the effects of military TBI given the wealth of evidence of biochemical changes in civilian and sports-related head injury. There is a wide range of studies that needto be conducted. To date there have been no studies conducted in severe military brain injury, for which MRS could have great prognostic value, nor in the acute stages of injury. This is likely due to the difficulty in conducting studies at first responder MRI facilities such as Landstuhl. However, subacute to chronic MRS studies could be readily conducted at the many MRI facilities across US Army and National Intrepid Centers of Excellence. Although the single MRSstudy in military personnel show that PTSD and other co-morbid conditions do not affect mTBI results, this issue needs to be examined in a larger cohort of subjects. It is also unclear ifthe controls were civilians or military, which may biasthe comparison. Furthermore, as the study utilized a moderate echo time, Glu and mI were not measured. These additional biomarkers, amongst others available to spectrally-sensitive MRS techniques, may have shown differences. Finally, as the study focused on the temporal lobes, there may also be other brain regions of interest, particularly posterior cingulate and parietal white matter which has shown high sensitivity and specificity for prognosis of severe TBI.
Overall, given the heterogeneity of mTBI, there still remains much work to be donebefore a viable diagnostic or prognostic tool can be utilized in the clinic, however the severe TBI work that has been done has demonstrated that metabolite measures can provide both diagnostic and more importantly prognostic measures. Current MRS methods such as single voxel spectroscopy and chemical shift imaging are clinically available across all MR manufacturers where an additional scan of 5–10 min can provide a wealth of information that can assist withoutcome measures in the severely injured, particularly for patients in comas. While the scientific evidence for the use of MRS in brain injury is strong, there remains the need for large, prospective clinical trials to confirm the clinical utility of MRS in severe brain injury.
Consensus as to how MRS data should be acquired and analyzed also remains elusive. Another knowledge gap is the paucity of studies that utilize a multimodal approach to determine how MRS can be complimentary and supportive of other imaging modalities such as DTI and volumetric sequences thus enabling a more complete picture of the pathophysiology of brain injury. This would be particularly important when evaluating mTBI within the context of comorbid conditions and where advanced MRS methods combined with other imaging methods can be used in conjunction to distinguish those changes specific to brain injury.
Task-based functional magnetic resonance imaging
Technique
In blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI), an increase in local CBF, blood volume, and oxygen extraction, i.e., hemodynamic response (HDR), is measured to infer neural activity during the performance of a task (Ogawa et al. 1992). Changes in the BOLD signal between two or more conditions are contrasted with each other in an attempt to isolate the brain regions involved in a wide variety of mental processes including perception, attention, language, memory, decision making, reasoning, emotion, and social cognition, among others. Stimuli are generally presented in a block or event-related design. In block designs, stimuli from several trials in one condition are presented while responses are collected. After one block is presented, another block of trials presenting stimuli from another condition are presented, and blocks are repeated on an alternating basis. In event-related studies, single trials from different conditions can be intermixed. The benefits of block design may be increased power and decreased time in the scanner. In rapid event-related designs, single trials can be separated by brief, varying intervals to prevent habituation or expectancy. Event-related designs may take longer to administer than block designs and are more complicated to analyze, but can permit the measurement of particular processes that may not be possible to isolate in a block design.
Current uses in military/veteran TBI studies
A handful of fMRI studies have been performed with subjects selected to have mTBI due to blast explosions in the Iraq and Afghanistan wars, and they primarily investigated alteration in regions involved in emotion and control of cognitive processes (Matthews et al. 2011a, 2011b; Fischer et al. 2014; Scheibel et al. 2012; Newsome et al. 2015). In one of the first fMRI studies investigating blast-related TBI, Matthews et al. (2011a) investigated whether emotion processing, in particular fear, was altered in subjects who also had major depressive disorder (MDD), compared to subjects with blast-related TBI but not MDD (Matthews et al. 2011a). Subjects viewed alternating blocks of emotional faces or shapes. In both conditions, a single target item (face or shape) was presented above two other faces or shapes, respectively, and subjects were asked to indicate which of the two items matched the target item. This emotion processing task had been previously validated to elicit amygdala activation in healthy volunteers (Hariri et al. 2002). The groups did not differ in the accuracy or reaction time with which they performed the task. Compared to the subjects with blast-related TBI alone, subjects with both TBI and MDD demonstrated increased activation in the amygdala bilaterally during fear processing, but decreased activation in a cognitive control region (e.g., dorsolateral prefrontal cortex (DLPFC)). The results suggested enhanced fear processing coupled with decreased ability to draw on neural sources required to regulate attention.
To measure cognitive control after concussion due to blast, Matthews et al. (2011b) presented an event-related task designed to elicit a prepotent manual response that had to be withheld (Matthews et al. 2011b), the stop signal task (Aron et al. 2003; Logan et al. 2000). In the task, either an “X” or an “O” was presented on screen, and subjects had to press a particular button when a letter appeared, but withhold their responses if a tone sounded, which occurred on 25 % of the trials. Trials were considered difficult if the tone was presented very close to (within 100–200 ms of) the average reaction time of the subject (determined in a session prior to the scan), and easy if the tone was presented 300–500 ms before the average reaction time of the subject. Both groups performed similarly in terms of accuracy and reaction time. There were also no group differences in activation for the difficult trials, but for the easy trials, Veterans who had experienced LOC demonstrated less activation in the ventromedial prefrontal cortex (VMPFC) than Veterans who had AOC. The voxel of peak activation in the VMPFC was in the middle frontal gyrus. The Veterans with LOC also demonstrated a positive correlation between brain activation in the VMPFC and somatic symptoms. The authors suggested that both the reduced activation and the correlation between activation and symptoms are related to diminished self-awareness subsequent to LOC.
In a separate study designed to measure conflict resolution, Scheibel et al. (2012) presented a stimulus–response compatibility task to subjects with blast-related mTBI an average of 2.6 years after their most recent blast exposure (Scheibel et al. 2012). In this rapid event-related design, subjects with and without mTBI viewed an arrow in the middle of a screen and were asked to press a button consistent with the direction the arrow pointed if the arrow were blue, or press a button inconsistent with the direction the arrow pointed if the arrow were red. As in the stop signal task, to create the expectation of a prepotent response, the red arrows were presented on only a small proportion of the trials. Both groups performed with similar accuracy rates. Relative to the Veterans without mTBI, the Veterans with mTBI demonstrated increased activation in the anterior cingulate cortex, medial prefrontal cortex, and regions associated with visual attention and spatial processing, and activation in these areas was augmented after covarying for measures of depression, PTSD, and reaction time (Scheibel et al. 2012). Please see Fig. 5. The authors concluded that the increased activation is consistent with previous investigations of this task in civilians with moderate to severe TBI (Scheibel et al. 2007, 2009) and may be compensatory, or reflect inefficient processing resulting from deafferentiation due to diffuse axonal injury.
Fig. 5.
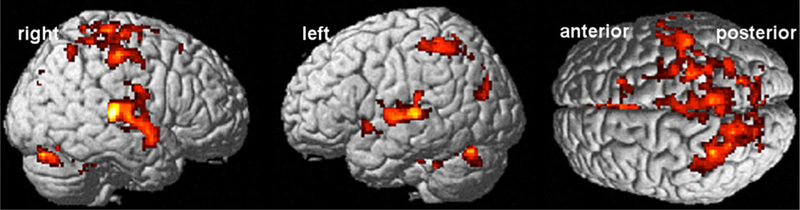
Subjects with blast mTBI who performed a stimulus–response compatibility task demonstrated greater activation than subjects who had not been exposed to blast, a pattern which was augmented after covarying for PTSD and depression symptoms and reaction time
Fischer et al. (2014) employed the stop signal task in an investigation of how blast-related mTBI might differ from civilian mTBI, investigating long term changes more than 4 years after blast exposure (Fischer et al. 2014). There was no effect of TBI in the performance of the task, although military groups demonstrated slower reaction time than the civilians. There were no group differences in accuracy during performance of the task. When correctly inhibiting their responses, the military TBI group had alterations in activation similar to those in the civilian TBI group, with decreased activation in medial and middle frontal gyri, anterior cingulate, middle temporal gyrus, and precuneus. As some of these regions are involved in the Default Mode Network (Raichle et al. 2001) (see below), and the inhibition condition of the stop signal task has been previously shown to correlate with the functional connectivity of the DMN (Zhang and Li 2012), the authors suggested this pattern may indicate that TBI of any etiology may disrupt DMN function during successful inhibition. However, the two mTBI groups did differ during correct inhibitions in anterior cingulate and orbital gyrus, with increased activation observed in the blast mTBI group. Further, when failing to inhibit, the blast-related mTBI group demonstrated increased activation (relative to a military control group) in the caudate nucleus and cerebellum, whereas the civilian mTBI group demonstrated decreased activation (relative to a civilian control group). Covarying for education, post-concussion, PTSD, depression, and pain symptoms did not alter patterns of significance. The authors posited that the results could be due to different underlying patterns of white matter disruption in the two mTBI groups and suggested that the stop signal task during fMRI may provide a biomarker for detecting chronic mTBI due to blast.
Newsome and colleagues (Newsome et al. 2015) also investigated differences between civilian and military TBI in working memory in subjects that overlapped with those in Fischer et al. (2014). The Sternberg Item Recognition Task (Sternberg 1966) was used to measure subcomponent processes of working memory, and the authors reported altered activation during the encoding phase, when subjects had to introduce and establish a representation of the information in the brain. In an event-related design, subjects viewed a screen with one, three, or five letters for 1800 ms (encoding), which was followed by a crosshairs for 4300 ms (maintenance), followed by a single letter to which they had to respond whether or not had been in the initial screen of letters (retrieval). The blast group did not demonstrate a monotonic relationship between working memory set size and activation in the right caudate during encoding that was found in the other groups. For performance, all groups demonstrated the set size effect first reported by Sternberg (1966), where reaction time increased with the number of letters to be remembered, but the blast TBI group was overall slower than all of the other groups, and their accuracy was worse. The authors suggested that the SIRT might be sensitive in detecting group differences and that the disrupted caudate activation provided further support (cf. Fischer et al. 2014) that the basal ganglia may be vulnerable to blast injury.
Limitations, challenges, and future directions
Task performance during fMRI has suggested neural alterations that are apparent in subjects several aftter blast-related TBI. Additional work documenting longitudinal changes across the lifespan may yield important information on whether these changes are stable or if more widespread alteration might occur. fMRI studies reviewed here suggest alterations in regions involved in controlling emotion and cognitive responses, despite normal performance, suggesting that brain alterations may exist independent of performance decrements for these tasks. Could these brain changes eventually affect future performance? Determining whether altered brain function has implications for future performance of tasks that rely on the altered regions may be useful to understand if continued testing and monitoring of cognitive tasks would be worthwhile when decrements in cognitive performance are not detected in office testing. Relation of fMRI results to multiple types of structural imaging will provide additional insight into the nature of the damage and may also help to predict future change.
In addition, investigation with tasks measuring other processes would provide a broader knowledge base of the effects of mTBI, particularly in military populations. Candidate tasks could include domains known to be challenged in Service Members and Veterans, e.g., social cognition tasks may identify regions that are compromised in Veterans who experience difficulty relating and integrating with their families and community, which may interact with PTSD symptoms. Accounting for co-morbidities is important in understanding any specific effects of TBI; however, overlapping symptoms in TBI, PTSD, and depression can affect interpretation, and the field may have to adapt to the limitation of not being able to isolate TBI from PTSD or other co-morbidities in some circumstances and develop new approaches. If results of present experiments are replicated in well-characterized subjects (whether they have TBI only or both TBI and depression, for example), task performance during fMRI may have the potential to classify patients. Although a very preliminary suggestion, one region that may be a biomarker may be the anterior cingulate, which was implicated in the cognitive control studies reviewed here and would be relevant for emotional regulation. Once vulnerable regions are reliably identified, future therapies that target these regions during neural feedback (Sokunbi et al. 2014; LaConte et al. 2007; Yuan et al. 2014) may have success in normalizing activation.
Resting state fMRI and functional connectivity
Technique
During fMRI scanning without task performance, low frequency spontaneous fluctuations in BOLD activity show patterns of significant correlations between regions (Biswal et al. 1995), or functionally connected networks. The Default Mode Network (DMN) is one well-known example and includes portions of the medial prefrontal cortex, posterior cingulate cortex, medial temporal and lateral parietal lobes, with the anterior and posterior cingulate cortices serving as hubs (Raichle et al. 2001; Fox et al. 2005; Buckner et al. 2008). In addition, functional connectivity in the DMN is anticorrelated with networks involved in cognitive tasks, e.g., intraparietal sulcus (Fox et al. 2005).
One analytical approach is a seed-based method, in which signal fluctuations from a region of interest (seed) are correlated with other areas of the brain. Other analysis methods include independent components analysis (ICA) (Beckmann et al. 2005) and graph theoretic analysis (Sporns et al. 2004). ICA decomposes signal into distinct spatial and temporal components and is model-free, which may be an advantage for when datasets are not well typified. In graph theoretic analysis, brain networks are modeled in terms of graphs (e.g., nodes and connections) to characterize network features, such as distance between regions (i.e., path length) and the extent to which a node is connected with the rest of the network (global efficiency), both of which are examples of measures of network integration. Other examples of how network organization may be understood include density of local connections and degree of influence nodes have on the network. As well, graphs can be divided into modules, and connections between the modules can be investigated.
Current uses in military/veteran TBI studies
Functional connectivity has been reported to be altered in civilians with mTBI (Mayer et al. 2011; Stevens et al. 2012). In subacute mTBI (i.e., 4 to 5 months post-injury), functional connectivity within the DMN was decreased, while connectivity between the DMN and regions with which it might normally be anticorrelated (e.g., lateral prefrontal cortex) was increased (Mayer et al. 2011). Disruption of electrophysiological signal within the lateral frontal lobes and the white matter networks subserving them in OEF/OIF/OND Veterans with mTBI (Sponheim et al. 2011), suggests that anti-correlations in brain networks may be disrupted in Veterans with mTBI.
Using ICA, Vakhtin and colleagues (Vakhtin et al. 2013) reported changes in three ICA measurements, BOLD spatial maps, spectral power, and functional connectivity. TBI subjects were screened to have only blast-related TBI. Relative to controls, who were healthy civilians from Allen and colleagues (Allen et al. 2011), the mTBI group demonstrated decreased activity in left inferior temporal lobe and increased activity in temporal parietal junctions bilaterally (spatial map results), increased frequency in attention, frontal, and DMN compononents (spectral results), and reduced functional connectivity in six pairs of networks. However, using a seedbased approach, Robinson et al. reported that proximity to blast, rather than the presence of concussion symptoms, was associated with disruption to the DMN in Veterans (Robinson et al. 2015). In particular, functional connectivity between a region of the posterior cingulate cortex (i.e., seed) and somatosensory and pre-supplementary motor cortices was decreased in Veterans who had been within 10 m of a blast explosion, relative to Veterans who had been exposed to blast at greater distances. The Veterans who had been in close proximity to the blast had more TBIs than the other Veterans, but the same results were found after accounting for duration of LOC, as well as other factors such as time since deployment and pain. The authors suggested that effects of blast may be underestimated in cases where TBI is not found. Using graph theoretic analyses, Spielberg et al. (2015) reported in a group of veterans with mTBI of mixed etiology and co-morbid PTSD a negative relation between re-experiencing severity and local efficiency, such that greater re-experiencing severity was associated with less efficient communication in a network surrounding the caudate (Spielberg et al. 2015). Different methods of analysis, co-morbidities, and TBI etiologies may play a role in the different results across studies.
Studies with active duty military subjects have reported altered connectivity. Using an ICA approach, Nathan et al. reported increased connectivity within posterior regions of the DMN in Active Duty Service Members who had a maximum of one mTBI and were between 2 and 10 months post-injury without a PTSD diagnosis, relative to Active Duty Service Members with no history of TBI (Nathan et al. 2015). They also demonstrated increased connectivity in supplementary motor area and cerebellum. Using a graph theory approach, Han et al. reported altered connectivity between modules (Han et al. 2014). They measured functional connectivity in active duty military personnel who had been exposed to blasts in combat, but some subjects were diagnosed with TBI while others, the comparison control group, were not. One group of TBI subjects was scanned within 90 days of injury (the first TBI group), and another, validation, group was scanned 30 days after injury (the second TBI group), with follow-up scans for all subjects occurring 6–12 months after the initial scans. The authors observed decreased connectivity between modules in both TBI groups at the first scan. In the first TBI group, a similar pattern was observed at the second scan, although in fewer subjects. The second TBI group showed no significant differences at follow-up. The authors suggested that differences in medication and sleep duration may account for differences at the two time points. It was suggested that the decreased modular connectivity could be due to white matter damage associated with metabolic dysfunction or to the influence of undetected gray matter damage on blood flow. See Table 3 for a summary of participant characteristics, imaging parameters and design specifics, and results for task-related and resting state fMRI.
Table 3.
FMRI and functional connectivity studies reported in chronological order
| Author | TBI participant characteristics and comparison cohort |
TBI population (all from Iraq or Afghanistan wars) |
Time post-injury | Magnet strength and vendor |
MRI parameters | fMRI task or type of FC analysis |
Summary of findings |
|---|---|---|---|---|---|---|---|
| Task-based fMRI | |||||||
| Matthews et al. 2011a |
Patients (all): 27 mTBI (27M,0F); 15 with LOC and 12 with AOC Age: LOC group: 26.9±5.6 years AOC group: 29.0±5.0 year |
Veterans | Mean years since most severe concussion LOC group: 3.2 years (SD=1.37) AOC group: 3.58 years (SD=1.31) |
3T; GE Signa EXCITE | T2* weighted echoplanar imaging, TR/TE=2000/32, FOV=230×230 mm, 64×64 matrix, 30 2.6 mm axial slices with a 1.4 mm gap. One run, 256 scans, 512 s |
Stop Signal Task Fast event-related |
No group differences in activation in the difficult trials. In easy trials, Veterans with LOC showed less activation in the VMPFC than Veterans who had AOC. Veterans with LOC also demonstrated a positive relation between brain activation in the VMPFC and somatic symptoms. |
| Matthews et al. 2011b |
Patients (all): 22 mTBI (22M,0F); including 11 with MDD and 11 without MDD Age: MDD group: 26.8 years, range=22–45 Non-MDD group: 30.3 years, range=22–47 |
Veterans | Mean years since most severe blast MDD: 2.8 (SD=1.0) Non-MDD: 3.3 (SD=1.1) |
3T; GE Signa EXCITE | T2* weighted echoplanar imaging, TR/TE=2000/32, FOV=230×230 mm, 64×64 matrix, 30 2.6 mm axial slices with a 1.4 mm gap. One run, 256 scans |
Emotional face matching task Block design |
Compared to the subjects with blast-related TBI alone, subjects with both TBI and MDD demonstrated increased activation in the amygdala bilaterally during fear processing, but decreased activation in a cognitive control region (e.g., DLPFC). |
| Scheibel et al. 2012 | 15 TBI (15M,0F) Age: 28.7±6.0 year, median=26.0 15 Veterans who were not exposed to blast and reported no TBI, including five had an orthopedic injury during deployment (14M,1F) Age: 30.9±5.6 years; median=33.0 |
Veterans and Active Duty Service Members | Mean number of days since most recent blast-related TBI 963.9 (333.2) |
3T; Philips Achieva | T2* weighted singleshot gradient-echo EPI; TR/TE=1700/30 ms; FA=73°;FOV=240 mm; 64×64 matrix; 3.75 mm thickness with 0.5 mm gap; 32 axial slices; 160 volumes. SENSE factor 2.0. 3 runs, each 244 s | Stimulus–response compatibility task Rapid-presentation, stochastic event-related |
Relative to the Veterans without mTBI, the Veterans with mTBI demonstrated increased activation in the anterior cingulate cortex, medial prefrontal cortex, and regions associated with visual attention and spatial processing. |
| Fischer et al. 2014 |
Patients: 21 MilTBI (20M,1F); Age: 28.3 ±4.6 years 21 CivTBI (Civilians with mild to moderate TBI; 19M,2F); Age: 26.2±4.8 years Control: 22 MilCon (Veterans without exposure to blast or history of TBI; 21M,1F) Age: 29.7±5.6 years 23 CivCon (Civilians with extracranial injury; 23M,0F) Age: 27.3 ±4.5 years |
Veterans | Mean months since most severe TBI MilTBI: 52.9 (17.9) CivTBI: 29.7 (16.1) |
3T; Siemens TIM Trio | Gradient-echo EPI; TR/TE=2800/29 ms; FA=80°; FOV=256; matrix=128×128; 31 4-mm thick contiguous axial slices, in-plane resolution=2×2-mm); whole-brain; 12-channel receive-only head array. 2 runs, each 736 s (263 volumes per run) |
Stop Signal Task Event-related |
During correct inhibitions, the blast mTBI group demonstrated increased activation in ACC and orbital gyrus. During incorrect inhibitions, the blast mTBI group demonstrated increased activation (relative to a military control group) in the caudate nucleus and cerebellum, whereas the civilian mTBI group demonstrated decreased activation (relative to a civilian control group). There was no effect of TBI in the performance of the task, although military groups demonstrating poorer performance than the civilians. |
| Newsome et al. 2015 |
Patients: 25 MilTBI with mild (23)to moderate (2) TBI (21 M,4F); Age: 29.6 (6.01) 25 CivTBI (Civilians with mild (23) to moderate (2) TBI; 24M, IF); Age: 27.4 (6.7) Control: 25 MilCon (Veterans without exposure to blast or history of TBI; 25M,0F) Age: 29.9 (5.5) 25 CivCon (Civilians with extracranial injury; 25M, OF) Age: 27.3 (5.8) |
Veterans | Mean months since most severe TBI MilTBI: 50.1 (18.0) CivTBI: 27.1 (15.0) |
3T; Siemens TIM Trio | Gradient-echo EPI; TR/TE=2800/29 ms; FA=80°; FOV=256; matrix=128×128; 31 4-mm thick contiguous axial slices, in-plane resolution=2×2-mm); who le-brain; 12-channel receive-only head coil 3 runs, each 585 s (209 volumes per run) | Sternberg Item Recognition Task Event-related |
All groups except the blast group demonstrated a monotonic relationship between working memory set size and activation in the right caudate during encoding. For performance, all groups demonstrated the set size effect first reported by Sternberg (1966), where reaction time increased with the number of letters to be remembered, but the blast TBI group was overall slower than all of the other groups, and their accuracy was worse. |
| Resting state | |||||||
| Vakhtin et al. 2013 |
Patients: 13 blast-only TBI (13M, OF) Age: 34.3 years, (SD=6.6) Control: 50 healthy civilian adults from Allen et al. (2011). (50M, OF) Age: 29.7 years, (SD=8.4) |
Veterans | Not provided | 3T; Siemens Trio | T2*-weighted gradient-echo EPI; TR/TE=2000/29 ms; FA=75°; FC)V=240; matrix=64 × 64; distance factor=30 %, voxel size=3.8×3.8×3.5 1 run: 5 min, 34 s (167 volumes total) |
Instructions to fixate eyes on crosshairs, relax and think of nothing in particular (eyes open) ICA to measure BOLD spatial maps, spectral power, and FC |
Relative to controls, the mTBI group demonstrated decreased activity in left inferior temporal lobe and increased activity in temporal parietal junctions bilaterally (spatial map results), increased frequency in attention, frontal, and DMN compononents (spectral results), and reduced FC in six pairs of networks (FC results). |
| Han et al. 2014 |
Patients: 63 TBI Cohort I (63M, OF) (54 in analysis) Age: 19–44 years (median=24) 40 TBI Cohort 2 (37M, 3F) (38 in analysis) Age: 19–44 years (median=23) Controls: 21 active duty military personnel with injuries other than TBI (20M, IF) (14 in analysis) Age: 19–49 (median 29) Subjects scanned twice with some drop-out. 55 datasets removed after QA. |
Active Duty Service Members |
Range and median days between injury and initial scan Cohort 1: 0–90, 14 Cohort 2: 0–30, 7 |
1.5 T; Siemens Magnetom Avanto |
TR/TE=2500/50 ms; FA=90°;FOV=25.6×25.6 cm; matrix=64×64; 30 axial slices (4.0 mm thick) covering whole cerebrum; 165 volumes. T2 *-weighted blipped EPI sequence 12-channel head coil 3 runs (each 412.5 s) |
No instructions to keep eyes open or closed or to stay awake. Graph theoretic analysis |
Decreased connectivity between modules in both TBI groups at the first scan, with a similar, but diminished pattern found at Time 2 in Cohort 1. Cohort 2 did not show the same pattern at Time 2. |
| Robinson et al. 2015 | 139 participants from TRACTS after exclusions (114M, based on 134 subjects with complete neuropsychological data) Age: 33.0±8.6 years; range = 19–62, | Active Duty Service Members and Veterans; 5 % of sample had not deployed | Months since deployment 31.9±26.2 |
3T; Siemens TIM Trio | Gradient echo EPI;TR/TE=3000/30 ms; FA=90°; 3 × 3 × 3.75 mm; 38 slices. 2 runs, 6 min per run |
Instructions to keep eyes open and stay awake Seed-based |
Proximity to blast, rather than the presence of concussion symptoms, associated with disruption to the DMN. |
| Nathan et al. 2015 | 15 TBI (15M) Age: 25.6±4 years 12 Active Duty Service Members without TBI age matched to TBI group (9M, 3F) Age: 26.4±5.8 years |
Active Duty Service Members | Days since injury 147.21±87.19 |
3T;GE | EPI, sagittal plane, TE/TR =25/2000 ms, FA=60°; FOV=240× 240mm; matrix size 64×64, 3.75×3.75×4 mm 1 run, 6 min | Instructions to keep eyes closed Dual-regression ICA |
Increased connectivity within posterior regions of the DMN and between supplementary motor area and cerebellum. |
| Spielberg et al. 2015 | 208 Veterans, 63 % of whom experienced TBI. Of the 63 % with TBI, TBI was due to blast, other military exposure, and pre-and post-deployment accidents. 52 % of sample met criteria for PTSD. |
Veterans | Not provided | 3T; Siemens TIM Trio | EPI; TR/TE=3000/30 ms,3×3×3 mm 2 runs; time per run=360 s |
Instructions to remain still with eyes open | A negative relation between re experiencing severity and local efficiency was observed, such that greater re-experiencing severity was associated with less efficient communication in a network surrounding the caudate. |
3T 3 Tesla; ACC anterior cingulate cortex; AOC Alteration of consciousness; DLPFC dorsolateral prefrontal cortex; DMN Default Mode Network; EPI echoplanar imaging; FC Functional Connectivity; ICA Independent Component Analysis; LOC Loss of consciousness; MDD Major Depressive Disorder; civCon Civilian Controls; civTBI Civilian TBI; FA flip angle; FOV field of view; milCon Military Controls; milTBI Military TBI; min minutes; mm millimeter; MRI magnetic resonance imaging; ms milliseconds; s seconds; SD standard deviation; TBI traumatic brain injury; TE echo time; TR repetition time; TRACTS Translational Research Center for TBI and Stress Disorders; VMPFC ventromedial prefrontal cortex
Limitations, challenges, and future directions
Little data exist on the functional connectivity of Service Members and Veterans exposed to blast, and a challenge will be toreplicate current results and build upon them with studies that further explore the nature of network disruption, using additional approaches and investigating additional regions. Accounting for different findings obtained with different methodologies will also be a challenge. Given the many types of analysis methods (some not covered here) and networks, meta-analyses will be critical in understanding fundamental changes after mTBI in the military, or even after exposure to blast without mTBI. The complication of heterogeneity in the locations impacted by TBI inherent in civilian TBI datasets is present in blast-related TBI studies with potential variability in overpressure effects and is compounded by type of injury as blunt force mTBI commonly accompanies blast mTBI. However, the prominence of resting state studies in the civilian literature suggests the field will be able to rise to the challenge, producing numerous datasets that, given data depositories such as the Federal Interagency Traumatic Brain Injury Research (FITBIR) informatics system, will allow for a variety of analyses. Many of the limitations inherent in task-based fMRI will also apply, e.g., comorbidities may play a confounding role, and relation to structural imaging will aid in better characterizing alterations.
Positron emission tomography
Technique
Positron emission tomography (PET) is a clinical imaging method that allows for the visualization of biological processes occurring at a cellular and/or molecular level. This approach involves the intravenous (IV) injection of a radiopharmaceutical followed by the imaging of this agent using a PET scanner that is capable of detecting picomolar concentrations of radiotracer uptake. Radiopharmaceuticals are imaging agents that are produced by coupling of a biologically active molecule labeled with a radioisotope that undergoes positron decay, decay, most commonly, 18F. Emitted positrons undergo annihilation to form two high-energy photons that travel in opposing directions. Coincident detection of high energy photons by a PET scanner is key to the high signal-to-noise and anatomical localization achieved by PET as compared to other nuclear imaging approaches such as single photon emission computed tomography (SPECT) or 2D planar gamma imaging. The biologically active component of a radiopharmaceutical is responsible for its targeting to locations of interest. Although a number of radiopharmaceuticals have been formulated and assessed experimentally in recent years, the majority of PET scans performed in the clinical setting utilize the tracer 18F-fluorodeoxyglucose (18F-FDG) (Stocker et al. 2014; Byrnes et al. 2014; Selwyn et al. 2013; Garcia-Panach et al. 2011; Zhang et al. 2010). 18F-FDG is a nonmetabolizable glucose analog that is transported via standard glucose uptake pathways into metabolically active cells, where it is subsequently localized through PET scanning techniques.
Current uses in military/veteran TBI studies
PET imaging has been applied in a limited number of studies to characterize changes occurring in military service members that have experienced a TBI. All available studies have utilized 18F-FDG to characterize cerebral metabolic correlates of TBI. Peskind et al. studied 12 Iraqi war Veterans with persistent post-concussive symptoms following exposure to at least one explosive blast (Peskind et al. 2011). Each had mTBI a defined by the American Congress of Rehabilitation Medicine (ACRM) criteria. 18F-FDG PET scanning was performed that revealed decreased uptake in the cerebellum, vermis, pons, and medial temporal lobe as compared to 12 healthy controls. Mendez et al. assessed 12 Veterans from conflicts in Iraq or Afghanistan who were exposed to “pure” blast resulting in a mTBI and compared them to 12 Veterans who experienced blunt force mTBI (Mendez et al. 2013). 18F-FDG scanning revealed hypometabolism in the right superior parietal region in the “pure” blast group as compared to blunt force controls. Stocker et al. studied a group of 14 Veterans with a history of direct blast exposure and/or mild TBI compared with 11 Veterans without TBI (Stocker et al. 2014). 18F-FDG PET scanning demonstrated cerebral hypometabolism in the amygdala, hippocampus, parahippocampal gyrus, thalamus, insula, uncus, culmen, visual association cortices, and midline medial frontal cortices in the blast exposed/mTBI group as compared with healthy Veteran controls. Petrie et al. assessed 34 veterans with a history of blast and/or impact related mild TBI as compared with 18 healthy controls (Petrie et al. 2014). 18F-FDG PET scanning demonstrated reduced glucose uptake in the parietal, somatosensory, and visual cortices as compared to healthy controls.
Limitations, challenges and future directions
As a contemporary clinical imaging modality, PET has great potential for the neuroimaging of biological processes associated with TBI that may help to refine a diagnosis, inform prognosis, and precisely guide patient and pathology specific therapy. However the current clinical application of PET in the imaging of TBI is largely limited to the visualization of relative changes in cerebral metabolism using F-FDG (Stocker et al. 2014; Byrnes et al. 2014; Selwyn et al. 2013; Garcia-Panach et al. 2011; Zhang et al. 2010). Although an array of radiotracers are under development and study for the neuroimaging of TBI, the utility of these agents in helping to improve management of the TBI patient will require additional investigation.
There are a number of potential uses for PET in military studies that are currently under development in both civilian and military populations. Mitsis et al. recently published the first report demonstrating uptake of the paired helical filament (PHF) tau ligand 18F-T807 in a former professional football player with clinically probable CTE (Mitsis et al. 2014). Imaging of this same subject using the amyloid imaging agent 18F-florbetapir revealed that no detectablecerebral amyloidosis was present, thereby excluding Alzheimer’s disease (AD) as the cause of his cognitive decline. This report also demonstrated focal retention of 18F-florbetapir within regions of prior contusion in a separate a patient who had sustained an impact TBI 8–10 months prior to imaging (see Fig. 6). Although these results are compelling, larger trials are needed to further characterize this radiotracer and help to differentiate specific binding reflective of CTE from non-TBI related findings such as progressive age related tauopathy (PART) (Crary et al. 2014).
Fig. 6.
59 year old with TBI following a fall demonstrating increased uptake of 18F-florbetapir (arrows) and decreased uptake of 18F-FDG (arrows) within the region of a previous traumatic cerebral contusion to the occiput (Mitsis et al. 2014). Figure used with permission
Hong et al. demonstrated variable uptake of the amyloidimaging agent 11C-PiB in a case series of patients with various severities of TBI including mTBI (Hong et al. 2014). The importance of the Hong et al. study was the demonstration that acute amyloidosis could be cleared, at least in part, although some subjects showed cerebral amyloidosis up to 1 year post TBI. Kawai et al. (2013) demonstrated 11C-PiB uptake in 3 of 12 individuals with neuropsychological impairment following moderate to severe TBI (Kawai et al. 2013) For the imaging of TBI-associated inflammation, the first generation translocator protein (TSPO) ligand 11C– PK11195 has shown uptake in several studies of patients with TBI (Folkersma et al. 2009, 2011; Ramlackhansingh et al. 2011). Although the widespread adoption of this first generation agent has been limited by a requirement for 11C radiolabeling, necessity of correlative serum studies to account for the presence of metabolites, and generally poor binding affinity. However, early results from TBI studies utilizing second-generation 18F-labeled TSPO ligands have been quite promising (Coughlin et al. 2015). Limited studies utilizing the neurotransmitter receptor ligands C-flumazenil, C-MP4A, and 18F-GE179 have also demonstrated promising results in TBI patients and preclinical models. Although the above studies are encouraging, all reflect early findings in limited populations and should be validated with larger trials. Additionally, investments in radiotracer discovery are necessary to help expand the repertoire of radiopharmaceuticals available for study in the diagnosis and management of TBI. Special emphasis should be placed on postmortem correlation whenever possible.
Magnetic source imaging/magentoencepaholography
Technique
Magnetoencephalography (MEG) is a method of measuring magnetic flux on the surface of the head which is associated with underlying neuronal electrical currents produced between synapses or within the axons or dendrites of neurons. Similar to electroencephalogram (EEG) and evoked potential (EPs) recordings, MEG can be used to detect abnormalities in spontaneous brain activity (resting-state MEG recording procedures). Additionally, task-related MEG paradigms can also be used for localization and estimation of the order and time course for these signals and allow construction of images of brain activity, a process often referred to as magnetic source imaging (MSI).
Current uses in military/veteran TBI studies
Because of its high degree of both spatial (2–3 mm at cortical level) and temporal (<1 ms) resolution, MSI has been considered a potentially useful tool in TBI-related research (Bigler 2001), particularly for detection of abnormally reduced connectivity or delay in activation in mTBI where conventional MR findings are unrevealing (Lewine et al. 1999, 2007; Tarapore et al. 2013; da Costa et al. 2014) and where performance deficits on neuropsychological testing are not observed (da Costa et al. 2014). A recent study utilizing MEG found low frequency signal in patients both with and without blast-related mTBI, and the number of cortical regions that generated abnormal slow-waves correlated significantly with the total post-concussive symptom scores in symptomatic TBI patients (Huang et al. 2012). Subsequent data by the same group demonstrated significant correlation between MEG source magnitude and post-concussive symptoms scores in a group of 36 active duty military service members and OIF/OEF Veterans with mTBI caused by blast exposure during combat, where personality change symptoms positively correlated with MEG slow-wave generation in bilateral orbitofrontal and ventromedial prefrontal cortex, concentration difficulties and affective lability positively correlated with slow-wave generation in the right orbitofrontal cortex, and visual symptoms correlated with slow wave generation in the right fusiform gyrus (Huang et al. 2014). Another study utilizing resting-state MEG demonstrated that, in comparison to normal controls, Veterans with mTBI showed a lower Lempel-Ziv Complexity (LZC), a measure of system complexity that estimates the number of different patterns in a sequence, in various brain regions including the right anterior frontal area, bilateral frontal area, and bilateral parietal-temporal area. Moreover, significant correlations were observed between the LZC measures and neuropsychological measures of motor coordination and speed, visual perceptual skills, and reasoning ability (Luo et al. 2013).
Limitations, challenges and future directions
One of the most significant special considerations regarding the use of MEG/MSI involve access to the magnetometers or gradiometers necessary for data acquisition and specialized expertise required for data analysis. Second, although the temporal resolution of MEG is excellent, variations in signaling can be introduced through a number of extraneous sources (e.g., external stimulation, movement, unrelated mental activity, etc.); therefore, use of MEG/MSI in acute mTBI may be challenged by issues such as drowsiness, suboptimal cooperation by the subject, and states of altered consciousness. MSI is best used for measurement of surface cortical activity; therefore, brain regions such as the basal temporal or subcortical areas may be difficult to accurately measure depending on the modeling used. Certain medications, including some sedative neuroleptics and hypnotics, are known to increase delta-wave power (Niedermeyer 2005) and the influence of medication use should be considered in data interpretation. Finally, little is known regarding the long-term persistence or time course of MEG-related changes. Despite these challenges and limitations, MEG may have a future role in prognosis, prediction, monitoring recovery from TBI, and in evaluating the efficacy of interventions (Tarapore etal. 2013; Castellanos etal. 2011; Tormenti et al. 2012). Use of more automated and single-subject analysis in individual cases with mTBI is particularly appealing in the clinical management of these patients (Huang etal. 2014).
Quality control for neuroimaging studies
In addition to routine quality assurance (QA) required for MRI, it is important to note that advanced sequences, such as ones discussed in this review, require additional quality measures to aid in robust performance and uniformity across scanners. A phantom is a useful tool to measure the performance of the scanner since MR measurements of the phantom can be recorded and compared to the known parameters manufactured in the phantom. In QA procedures involving phantoms, it is important to note that not one phantom scan can assure the performance of every capability of the scanner, especially for performance on advanced acquisitions as seen in TBI studies. A large amount of work has been done by groups such as the Alzheimer’s Disease Neuroimaging Initiative (ADNI) and Biomedical Informatics Research Network (BIRN), to create robust phantom QA for ongoing studies. For example, the phantom provided by ADNI works well for monitoring geometric distortion of volumetric data (Maikusa et al. 2013). The phantom scans and analysis developed by BIRN works very well for monitoring of functional MRI data (Friedman and Glover 2006). These QA procedures can be combined given the needs of a certain study. For example, the Chronic Effects of Neurotrauma Consortium (CENC) integrates weekly ADNI and BIRN scans as part of the QA requirement to actively monitor any changes in scanner performance pertaining to volumetric and fMRI scans, since these scans, along with others, are critical scans used in the consortium. If measurements with the phantoms go over the quality control limits set, engineers can correct the issue and then a qualified medical physicist or MR scientist can confirm the repair and determine the effects the issue and repair may have on the data quality.
The complexity of the human brain and the advancements of MR techniques have created a challenge to develop phantoms that can assure that advanced MR techniques are working properly and that they will properly characterize the brain tissue and structure. Two good examples are DTI and ASL MR. The development of phantoms to mimic human brain tissue in diffusion and flow properties are an active area of research to aid in the uniform performance of MRI systems for these types of scans. Data gleaned from phantom scans will aid QA efforts as there are already a number of post-processing techniques to aid in correcting acquired images from artifacts such as geometric distortion in DTI (Gholipour et al. 2011; Ruthotto et al. 2012; Huang et al. 2008). Another QA option is to have volunteers scanned at every site and compare measurements to make sure it is consistent. However, some metrics such as CBF in a human volunteer can vary based on several factors which can introduce variability in the QA data.
A major challenge being addressed by the imaging community is the variation of MR techniques across different vendors or different models and software versions of scanners within a common vendor. In multi-institutional and/or multivendor studies, unwanted variability in data can possibly be introduced when different scanners are used. In large TBI trials such as Transforming Research and Clinical Knowledge in TBI (TRACK-TBI), ADNI and CENC trials, careful consideration is given in planning the protocols to make the sequences as consistent as possible across scanners. The parameters prescribed (i.e. TR, number of measurements for fMRI, etc.) must be strictly adhered to in order to maintain uniformity of the data across institutions and scanners. For longitudinal studies, the same strict adherence to the protocol should be given since the patient may be scanned with the same protocol multiple times and the same protocol may be necessary for every subject in the study. If possible, the same scanner with the same hardware (i.e. coil) should be used. Any upgrades should be monitored by a qualified medical physicist or MR scientist to help assure consistency within the study. Physicists and/or MR scientists should also be available to address any artifacts seen in these studies and help remedy the issue while data is being collected.
Conclusion
Advanced imaging techniques hold great promise in advancing the diagnosis and prognosis of both acute and chronic military TBI, particularly in cases where CT and conventional MR sequences are unrevealing. Additionally, these techniques may significantly advance our understanding of the natural course of recovery from TBI, increase our understanding of the potential contribution of trauma to later neurodegenerative disease, and may enable evaluation of pharmacologic and rehabilitative interventions. However, many of these imaging techniques require additional testing prior to their application in clinical practice, particularly for Active Duty Service Members and Veterans.
While the number of investigators examining the effects of TBI in military and Veteran populations using neuroimaging is rapidly increasing, there remain gaps in the application of some of the modalities discussed here in these important populations. In addition to limited access to these subjects in an early (acute) state of TBI and the logistics of performing studies in mobile populations, there are significant complexities surrounding diagnosis, lack of “objective” indicators of injury, and the presence of comorbidities frequently associated with mTBI. Additionally, as with advanced imaging across the field of TBI and other disease processes, current barriers for application in clinical practice include the lack of standardization in acquisition and analysis tools and a lack of well-considered normative data that could be applied. Additionally, while several studies are currently underway to assess the long-term effects of neurotrauma using imaging, there are, at present, few published studies that utilize data collected longitudinally. There is still a growing need for large, multi-site studies, and related issues persist regarding consistency in imaging acquisition parameters, policies and mechanisms for storing and distributing large amounts of imaging data between sites, and methods for improved quality assurance across centers. Additionally, further consideration is warranted regarding how to best apply appropriate control groups that are truly comparable in terms of demographic factors and exposure to stressors. Finally, significant gaps also remain in our understanding of and evidence for the pathology underlying imaging findings.
Despite these limitations, work is underway to more completely develop these techniques for use in military TBI and address issues including standardization of imaging parameters, development of a normative database containing the advanced imaging information, and increased utilization of advanced imaging techniques at major military medical facilities. Recent investigation has attempted to consider forms of analysis that may better account for the heterogeneity in the nature and location of injury foci as well as methods that may automate imaging analysis and allow for examination of larger samples. Finally, there is increased interest in the use of different imaging modalities used in the same subject to glean different information about the brain derived from one or more forms of imaging, which may inform findings and a more complete understanding of the kind of injuries in the brain. This is important as no one imaging technique provides complete information about the brain or brain injuries. The field of neuroimaging is rapidly advancing, and studies involving military TBI are an important force in driving innovations, which will improve our understanding and treatment not only of combat-specific TBI, but of TBI more generally. Understanding further the mechanisms underlying mTBI and understanding the course of illness using multimodal techniques will also lead to radiological evidence used for detecting and diagnosing mTBI rather than symptom reports which are less reliable and which are non-specific for mTBI. Being able to detect brain injury early may also lead to early interventions that might prevent the long term changes that are observed in a minority of patients who have experienced an mTBI and who continue to have persistent symptoms. Finally, being able to objectively quantify brain changes in mTBI makes it possible to use such measures to monitor the efficacy of treatments that will likely be developed for testing in the near future.
Acknowledgments
The authors recognize the support of the US Department of Veterans Affairs (EAW, BAT, SG, MRN, MES, SG), the VA MERIT review grant program (1I01RX000684–01A2: SG, 1I01RX001062–01A1: EAW, MRN, and 1 I01 RX000928: MES, SB), and VA SPIRE program (VA 1 I21RX001565 BAT, and VA 1 I21RX001608 MRN); the Department of Defense Office of the Congressionally Directed Medical Research Programs (CDMRP) (W81XWH-10–1-0835: APL; X81XWH-07-CC-CSDoD: MES, SB), the National Institutes of Health (R01-NS078337: APL, MES, SB), Telemedicine and Advanced Technology Research Center (TATRC) at the U.S. Army Medical Research and Material Command (USAMRMC; W81XWH-13–2-0025: DFT), United States Army Medical Research Acquisition Activity (USAMRAA; W81XWH-09–2-0160: JRS, SG), the Chronic Effects Neurotrauma Consortium (CENC; PT108802-SC106187 and 1W81XWH-13–2-0095), and the Alzheimer’s Drug Discovery Foundation (SG). We also wish to thank Rhonda O’Donovan for her assistance in manuscript preparation.
Footnotes
Conflict of interest The authors declare that they have no competing interests.
References
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, et al. (2011). A baseline for the multivariate comparison of resting-state networks. Frontiers in Systems Neuroscience, 5, 2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L, et al. (2014). Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Sprenger T, Naegelin Y, Reinhardt J, Kuster P, Hirsch JG, et al. (2015). Comparison between balanced steady-state free precession and standard spoiled gradient echo magnetization transfer ratio imaging in multiple sclerosis: methodical and clinical considerations. Neuroimage, 108, 87–94. doi: 10.1016/j.neuroimage.2014.12.045. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Inokuchi R, Gunshin M, Yahagi N, & Suwa H (2012). Diffusion tensor imaging studies of mild traumatic brain injury: a meta-analysis. [Meta-Analysis]. Journal of Neurology, Neurosurgery, and Psychiatry, 83(9), 870–876. doi: 10.1136/jnnp-2012-302742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, & Robbins TW (2003). Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. [Research Support, Non-U.S. Gov’t]. Nature Neuroscience, 6(2), 115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Ashwal S, Holshouser B, Tong K, Serna T, Osterdock R, Gross M, et al. (2004). Proton spectroscopy detected myoinositol in children with traumatic brain injury. Pediatric Research, 56(4), 630–638. doi: 10.1203/01.PDR.0000139928.60530.7D. [DOI] [PubMed] [Google Scholar]
- Ashwal S, Babikian T, Gardner-Nichols J, Freier MC, Tong KA, & Holshouser BA (2006). Susceptibility-weighted imaging and proton magnetic resonance spectroscopy in assessment of outcome after pediatric traumatic brain injury. [Review]. Archives of Physical Medicine and Rehabilitation, 87(12 Suppl 2), S50–S58. doi: 10.1016/j.apmr.2006.07.275. [DOI] [PubMed] [Google Scholar]
- Bagley LJ, McGowan JC, Grossman RI, Sinson G, Kotapka M, Lexa FJ, et al. (2000). Magnetization transfer imaging of traumatic brain injury. [Research Support, U.S. Gov’t, P.H.S.]. Journal of Magnetic Resonance Imaging, 11(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Barker JW, Han PK, Choi SH, Bae KT, & Park SH (2015). Investigation of inter-slice magnetization transfer effects as a new method for MTR imaging of the human brain. PloS One, 10(2), e0117101. doi: 10.1371/journal.pone.0117101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, & Pierpaoli C (1996). Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance. Series B, 111(3), 209–219. [DOI] [PubMed] [Google Scholar]
- Bauman RA, Ling G, Tong L, Januszkiewicz A, Agoston D, Delanerolle N, et al. (2009). An introductory characterization of a combat-casualty-care relevant swine model of closed head injury resulting from exposure to explosive blast. Journal of Neurotrauma, 26(6), 841–860. doi: 10.1089/neu.2009-0898. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Donnelly K, Peterson DR, Warner GC, Zhu T, & Zhong J (2013). The relation between posttraumatic stress disorder and mild traumatic brain injury acquired during Operations Enduring Freedom and Iraqi Freedom. [Research Support, U.S. Gov’t, Non-P.H.S.]. The Journal of Head Trauma Rehabilitation, 28(1), 1–12. doi: 10.1097/HTR.0b013e318256d3d3. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Beare R, Ditchfield M, Coleman L, Babl FE, Kean M, et al. (2013). Susceptibility weighted imaging and its relationship to outcome after pediatric traumatic brain injury. [Research Support, Non-U.S. Gov’t]. Cortex, 49(2), 591–598. doi: 10.1016/j.cortex.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, & Smith SM (2005). Investigations into resting-state connectivity using independent component analysis. [Comparative Study Research Support, Non-U.S. Gov’t]. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 360(1457), 1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendlin BB, Ries ML, Lazar M, Alexander AL, Dempsey RJ, Rowley HA, et al. (2008). Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.]. NeuroImage, 42(2), 503–514. doi: 10.1016/j.neuroimage.2008.04.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED (2001). Distinguished Neuropsychologist Award Lecture 1999. The lesion(s) in traumatic brain injury: implications for clinical neuropsychology. Archives of Clinical Neuropsychology, 16(2), 95–131. [PubMed] [Google Scholar]
- Bigler ED, Anderson CV, & Blatter DD (2002). Temporal lobe morphology in normal aging and traumatic brain injury. [Research Support, Non-U.S. Gov’t]. AJNR. American Journal of Neuroradiology, 23(2), 255–266. [PMC free article] [PubMed] [Google Scholar]
- Bigler ED, Abildskov TJ, Petrie J, Farrer TJ, Dennis M, Simic N, et al. (2013). Heterogeneity of brain lesions in pediatric traumatic brain injury. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Neuropsychology, 27(4), 438–451. doi: 10.1037/a0032837. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, & Hyde JS (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. [Research Support, U.S. Gov’t, P.H.S.]. Magnetic Resonance in Medicine, 34(4), 537–541. [DOI] [PubMed] [Google Scholar]
- Bouix S, Pasternak O, Rathi Y, Pelavin PE, Zafonte R, & Shenton ME (2013). Increased gray matter diffusion anisotropy in patients with persistent post-concussive symptoms following mild traumatic brain injury. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.]. PloS One, 8(6), e66205. doi: 10.1371/journal.pone.0066205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Freeman T, Kimbrell T, Cardwell D, & Komoroski R (2003). In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of former prisoners of war with and without posttraumatic stress disorder. [Comparative Study]. The Journal of Neuropsychiatry and Clinical Neurosciences, 15(3), 367–370. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network: anatomy, function, and relevance to disease. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’tReview]. Annals of the New York Academy of Sciences, 1124, 1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Budde MD, Janes L, Gold E, Turtzo LC, & Frank JA (2011). The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. [Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t]. Brain, 134(Pt 8), 2248–2260. doi: 10.1093/brain/awr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Shah A, McCrea M, Cullinan WE, Pintar FA, & Stemper BD (2013). Primary blast traumatic brain injury in the rat: relating diffusion tensor imaging and behavior. Frontiers in Neurology, 4, 154. doi: 10.3389/fneur.2013.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes KR, Wilson CM, Brabazon F, von Leden R, Jurgens JS, Oakes TR, et al. (2014). FDG-PET imaging in mild traumatic brain injury: a critical review. [Review]. Frontiers in Neuroenergetics, 5, 13. doi: 10.3389/fnene.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese E, Du F, Garman RH, Johnson GA, Riccio C, Tong LC, et al. (2014). Diffusion tensor imaging reveals white matter injury in a rat model of repetitive blast-induced traumatic brain injury. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.]. Journal of Neurotrauma, 31(10), 938–950. doi: 10.1089/neu.2013.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan MF, Freund P, Draganski B, Anderson E, Cappelletti M, Chowdhury R, et al. (2014). Widespread age-related differences in the human brain microstructure revealed by quantitative magnetic resonance imaging. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Neurobiology of Aging, 35(8), 1862–1872. doi: 10.1016/j.neurobiolaging.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos NP, Leyva I, Buldu JM, Bajo R, Paul N, Cuesta P, et al. (2011). Principles of recovery from traumatic brain injury: reorganization of functional networks. [Research Support, Non-U.S. Gov’t]. NeuroImage, 55(3), 1189–1199. doi: 10.1016/j.neuroimage.2010.12.046. [DOI] [PubMed] [Google Scholar]
- Cecil KM, Hills EC, Sandel ME, Smith DH, McIntosh TK, Mannon LJ, et al. (1998). Proton magnetic resonance spectroscopy for detection of axonal injury in the splenium of the corpus callosum of brain-injured patients. [Comparative Study Research Support, U.S. Gov’t, P.H.S.]. Journal of Neurosurgery, 88(5), 795–801. doi: 10.3171/jns.1998.88.5.0795. [DOI] [PubMed] [Google Scholar]
- Cernak I, & Noble-Haeusslein LJ (2010). Traumatic brain injury: an overview of pathobiology with emphasis on military populations. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S. Review]. Journal of Cerebral Blood Flow and Metabolism, 30(2), 255–266. doi: 10.1038/jcbfm.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamard E, Theoret H, Skopelja EN, Forwell LA, Johnson AM, & Echlin PS (2012). A prospective study of physician-observed concussion during a varsity university hockey season: metabolic changes in ice hockey players. Part 4 of 4. [Research Support, Non-U.S. Gov’t]. Neurosurgical Focus, 33(6), E4. doi:10.317½012.10.FOCUS12305. 1–7. [DOI] [PubMed] [Google Scholar]
- Chamard E, Lassonde M, Henry L, Tremblay J, Boulanger Y, De Beaumont L, et al. (2013). Neurometabolic and microstructural alterations following a sports-related concussion in female athletes. Brain Injury, 27(9), 1038–1046. doi: 10.3109/02699052.2013.794968. [DOI] [PubMed] [Google Scholar]
- Choe AS, Belegu V, Yoshida S, Joel S, Sadowsky CL, Smith SA, et al. (2013). Extensive neurological recovery from a complete spinal cord injury: a case report and hypothesis on the role of cortical plasticity. Frontiers in Human Neuroscience, 7, 290. doi: 10.3389/fnhum.2013.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BA, Inglese M, Rusinek H, Babb JS, Grossman RI, & Gonen O (2007). Proton MR spectroscopy and MRI-volumetry in mild traumatic brain injury. [Controlled Clinical Trial Research Support, N.I.H., Extramural]. AJNR. American Journal of Neuroradiology, 28(5), 907–913. [PMC free article] [PubMed] [Google Scholar]
- Corbo V, Salat DH, Amick MM, Leritz EC, Milberg WP, & McGlinchey RE (2014). Reduced cortical thickness in veterans exposed to early life trauma. [Research Support, U.S. Gov’t, Non-P.H.S.]. Psychiatry Research, 223(2), 53–60. doi: 10.1016/j.pscychresns.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Munro CA, Ma S, Yue C, Chen S, et al. (2015). Neuroinflammation and brain atrophy in former NFL players: an in vivo multimodal imaging pilot study. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Neurobiology of Disease, 74, 58–65. doi: 10.1016/j.nbd.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. (2014). Primary age-related tauopathy (PART): a common pathology associated with human aging. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Acta Neuropathologica, 128(6), 755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa L, Robertson A, Bethune A, MacDonald MJ, Shek PN, Taylor MJ, et al. (2014). Delayed and disorganised brain activation detected with magnetoencephalography after mild traumatic brain injury. Journal of Neurology, Neurosurgery, and Psychiatry. doi: 10.1136/jnnp-2014-308571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport ND, Lim KO, Armstrong MT, & Sponheim SR (2012). Diffuse and spatially variable white matter disruptions are associated with blast-related mild traumatic brain injury. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.]. NeuroImage, 59(3), 2017–2024. doi: 10.1016/j.neuroimage.2011.10.050. [DOI] [PubMed] [Google Scholar]
- Davenport ND, Lim KO, & Sponheim SR (2015). White matter abnormalities associated with military PTSD in the context of blast TBI. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-PH.S.]. Human Brain Mapping, 36(3), 1053–1064. doi: 10.1002/hbm.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoteaux M, Deriche R, Le Bihan D, Mangin JF, & Poupon C (2011). Multiple q-shell diffusion propagator imaging. [Research Support, Non-U.S. Gov’t]. Medical Image Analysis, 15(4), 603–621. doi: 10.1016/j.media.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Detre JA, Leigh JS, Williams DS, & Koretsky AP (1992). Perfusion imaging. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. Magnetic Resonance in Medicine, 23(1), 37–45. [DOI] [PubMed] [Google Scholar]
- Ding K, Marquez de la Plata C, Wang JY, Mumphrey M, Moore C, Harper C, et al. (2008). Cerebral atrophy after traumatic white matter injury: correlation with acute neuroimaging and outcome. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-PH.S.]. Journal of Neurotrauma, 25(12), 1433–1440. doi: 10.1089/neu.2008.0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dortch RD, Moore J, Li K, Jankiewicz M, Gochberg DF, Hirtle JA, et al. (2013). Quantitative magnetization transfer imaging of human brain at 7 T. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. NeuroImage, 64, 640–649. doi: 10.1016/j.neuroimage.2012.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi H, Wiseman N, Liu J, Wang W, Welch RD, O’Neil BJ, et al. (2015). Cerebral hemodynamic changes of mild traumatic brain injury at the acute stage. PloS One, 10(2), e0118061. doi: 10.1371/journal.pone.0118061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis DB, & Kindlmann G (2006). Orthogonal tensor invariants and the analysis of diffusion tensor magnetic resonance images. [Research Support, N.I.H., Extramural]. Magnetic Resonance in Medicine, 55(1), 136–146. doi: 10.1002/mrm.20741. [DOI] [PubMed] [Google Scholar]
- Farbota KD, Sodhi A, Bendlin BB, McLaren DG, Xu G, Rowley HA, et al. (2012). Longitudinal volumetric changes following traumatic brain injury: a tensor-based morphometry study. [Research Support, N.I.H., Extramural]. Journal of the International Neuropsychological Society, 18(6), 1006–1018. doi: 10.1017/S1355617712000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BL, Parsons M, Durgerian S, Reece C, Mourany L, Lowe MJ, et al. (2014). Neural activation during response inhibition differentiates blast from mechanical causes of mild to moderate traumatic brain injury. [Comparative Study Randomized Controlled Trial]. Journal of Neurotrauma, 31(2), 169–179. doi: 10.1089/neu.2013.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkersma H, Boellaard R, Vandertop WP, Kloet RW, Lubberink M, Lammertsma AA, et al. (2009). Reference tissue models and blood–brain barrier disruption: lessons from (R)-[11C]PK11195 in traumatic brain injury. [Research Support, Non-U.S. Gov’t]. Journal of Nuclear Medicine, 50(12), 1975–1979. doi: 10.2967/jnumed.109.067512. [DOI] [PubMed] [Google Scholar]
- Folkersma H, Boellaard R, Yaqub M, Kloet RW, Windhorst AD, Lammertsma AA, et al. (2011). Widespread and prolonged increase in (R)-(11)C-PK11195 binding after traumatic brain injury. [Research Support, Non-U.S. Gov’t]. Journal of Nuclear Medicine, 52(8), 1235–1239. doi: 10.2967/jnumed.110.084061. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, & Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. [Comparative Study Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, P.H.S.]. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Shin J, Jagannathan K, Suh JJ, Detre JA, O’Brien CP, et al. (2012). Acute baclofen diminishes resting baseline blood flow to limbic structures: a perfusion fMRI study. [Research Support, N.I.H., Extramural]. Drug and Alcohol Dependence, 125(1–2), 60–66. doi: 10.1016/j.drugalcdep.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman TW, Cardwell D, Karson CN, & Komoroski RA (1998). In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combat-related posttraumatic stress disorder. [Clinical Trial Controlled Clinical Trial]. Magnetic Resonance in Medicine, 40(1), 66–71. [DOI] [PubMed] [Google Scholar]
- Friedman L, & Glover GH (2006). Report on a multicenter fMRI quality assurance protocol. [Research Support, N.I.H., Extramural Review]. Journal ofMagnetic Resonance Imaging, 23(6), 827–839. doi: 10.1002/jmri.20583. [DOI] [PubMed] [Google Scholar]
- Gandy SE, Snow RB, Zimmerman RD, & Deck MD (1984). Cranial nuclear magnetic resonance imaging in head trauma. [Case Reports]. Annals of Neurology, 16(2), 254–257. doi: 10.1002/ana.410160217. [DOI] [PubMed] [Google Scholar]
- Garcia-Panach J, Lull N, Lull JJ, Ferri J, Martinez C, Sopena P, et al. (2011). A voxel-based analysis of FDG-PET in traumatic brain injury: regional metabolism and relationship between the thalamus and cortical areas. Journal of Neurotrauma, 28(9), 1707–1717. doi: 10.1089/neu.2011.1851. [DOI] [PubMed] [Google Scholar]
- Gardner A, Iverson GL, & Stanwell P (2014). A systematic review of proton magnetic resonance spectroscopy findings in sport-related concussion. [Review]. Journal of Neurotrauma, 31(1), 1–18. doi: 10.1089/neu.2013.3079. [DOI] [PubMed] [Google Scholar]
- Garnett MR, Blamire AM, Corkill RG, Cadoux-Hudson TA, Rajagopalan B, & Styles P (2000). Early proton magnetic resonance spectroscopy in normal-appearing brain correlates with outcome in patients following traumatic brain injury. [Clinical Trial Research Support, Non-U.S. Gov’t]. Brain, 123(Pt 10), 2046–2054. [DOI] [PubMed] [Google Scholar]
- Gasparovic C, Yeo R, Mannell M, Ling J, Elgie R, Phillips J, et al. (2009). Neurometabolite concentrations in gray and white matter in mild traumatic brain injury: an 1H-magnetic resonance spectroscopy study. Journal of Neurotrauma, 26(10), 1635–1643. doi: 10.1089/neu.2009-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholipour A, Kehtarnavaz N, Scherrer B, & Warfield SK (2011). On the accuracy of unwarping techniques for the correction of susceptibility-induced geometric distortion in magnetic resonance Echo-planar images. [Research Support, N.I.H., Extramural]. Conference of the IEEE Engineering in Medicine and Biology Society, 2011, 6997–7000. 10.1109/IEMBS.2011.6091769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz KM, & Valovich McLeod TC (2011). Pediatric sports-related concussion. [Review]. PM&R, 3(4), 353–364. doi: 10.1016/j.pmrj.2010.12.006. quiz 364. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Raza W, Wu B, & Kou Z (2013). The presence of venous damage and microbleeds in traumatic brain injury and the potential future role of angiographic and perfusion magnetic resonance imaging In Kreipke CW & Rafols JA (Eds.), Cerebral blood flow, metabolism, and head trauma (pp. 75–94). New York: Springer. [Google Scholar]
- Han K, Mac Donald CL, Johnson AM, Barnes Y, Wierzechowski L, Zonies D, et al. (2014). Disrupted modular organization of resting-state cortical functional connectivity in U.S. military personnel following concussive ‘mild’ blast-related traumatic brain injury. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.]. NeuroImage, 84, 76–96. doi: 10.1016/j.neuroimage.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, & Weinberger DR (2002). The amygdala response to emotional stimuli: a comparison of faces and scenes. [Clinical Trial Comparative Study Research Support, U.S. Gov’t, P.H.S.]. NeuroImage, 17(1), 317–323. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Cooper E, Dowell NG, Keramida G, Voon V, Critchley HD, et al. (2014). Quantitative magnetization transfer imaging as a biomarker for effects of systemic inflammation on the brain. Biological Psychiatry. doi: 10.1016/j.biopsych.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartkamp NS, van Osch MJ, Kappelle J, & Bokkers RP (2014). Arterial spin labeling magnetic resonance perfusion imaging in cerebral ischemia. [Research Support, Non-U.S. Gov’t Review]. Current Opinion in Neurology, 27(1), 42–53. doi: 10.1097/WCO.0000000000000051. [DOI] [PubMed] [Google Scholar]
- Haseler LJ, Arcinue E, Danielsen ER, Bluml S, & Ross BD (1997). Evidence from proton magnetic resonance spectroscopy for a metabolic cascade of neuronal damage in shaken baby syndrome. [Research Support, Non-U.S. Gov’t]. Pediatrics, 99(1), 4–14. [DOI] [PubMed] [Google Scholar]
- Helmick KM, Spells CA, Malik SZ, Davies CA, Marion DW, Hinds SR (2015). Traumatic brain injury in the US military: epidemiology and key clinical and research programs. Brain Imaging Behav. doi: 10.1007/s11682-015-9399-z. [DOI] [PubMed] [Google Scholar]
- Henry LC, Tremblay S, Leclerc S, Khiat A, Boulanger Y, Ellemberg D, et al. (2011). Metabolic changes in concussed American football players during the acute and chronic post-injury phases. [Research Support, Non-U.S. Gov’t]. BMC Neurology, 11, 105. doi: 10.1186/1471-2377-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington HP, Hamid H, Kulas J, Ling G, Bandak F, de Lanerolle NC, et al. (2014). MRSI of the medial temporal lobe at 7 T in explosive blast mild traumatic brain injury. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Magnetic Resonance in Medicine, 71(4), 1358–1367. doi: 10.1002/mrm.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshouser BA, Tong KA, & Ashwal S (2005). Proton MR spectroscopic imaging depicts diffuse axonal injury in children with traumatic brain injury. AJNR. American Journal of Neuroradiology, 26(5), 1276–1285. [PMC free article] [PubMed] [Google Scholar]
- Hong YT, Veenith T, Dewar D, Outtrim JG, Mani V, Williams C, et al. (2014). Amyloid imaging with carbon 11-labeled Pittsburgh compound B for traumatic brain injury. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. JAMA Neurology, 71(1), 23–31. doi: 10.1001/jamaneurol.2013.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Ceritoglu C, Li X, Qiu A, Miller MI, van Zijl PC, et al. (2008). Correction of B0 susceptibility induced distortion in diffusion-weighted images using large-deformation diffeomorphic metric mapping. [Research Support, N.I.H., Extramural]. Magnetic Resonance Imaging, 26(9), 1294–1302. doi: 10.1016/j.mri.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MX, Nichols S, Robb A, Angeles A, Drake A, Holland M, et al. (2012). An automatic MEG low-frequency source imaging approach for detecting injuries in mild and moderate TBI patients with blast and non-blast causes. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.]. NeuroImage, 61(4), 1067–1082. doi: 10.1016/j.neuroimage.2012.04.029. [DOI] [PubMed] [Google Scholar]
- Huang MX, Nichols S, Baker DG, Robb A, Angeles A, Yurgil KA, et al. (2014). Single-subject-based whole-brain MEG slow-wave imaging approach for detecting abnormality in patients with mild traumatic brain injury. [Research Support, Non-U.S. Gov’t]. NeuroImage: Clinical, 5, 109–119. doi: 10.1016/j.nicl.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JV, Wilde EA, Tong KA, & Holshouser BA (2012). Emerging imaging tools for use with traumatic brain injury research. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S. Review]. Journal of Neurotrauma, 29(4), 654–671. doi: 10.1089/neu.2011.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac L, Main KL, Soman S, Gotlib IH, Furst AJ, Kinoshita LM, et al. (2015). The impact of depression on Veterans with PTSD and traumatic brain injury: a diffusion tensor imaging study. Biological Psychology, 105, 20–28. doi: 10.1016/j.biopsycho.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Ito R, Mori S, & Melhem ER (2002). Diffusion tensor brain imaging andtractography. Neuroimaging Clinics of North America, 12(1), 1–19. [DOI] [PubMed] [Google Scholar]
- Johnson B, Gay M, Zhang K, Neuberger T, Horovitz SG, Hallett M, et al. (2012). The use of magnetic resonance spectroscopy in the subacute evaluation of athletes recovering from single and multiple mild traumatic brain injury. [Research Support, N.I.H., Extramural]. Journal of Neurotrauma, 29(13), 2297–2304. doi: 10.1089/neu.2011.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge RE, Acion L, White T, Tordesillas-Gutierrez D, Pierson R, Crespo-Facorro B, et al. (2012). White matter abnormalities in veterans with mild traumatic brain injury. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.]. The American Journal of Psychiatry, 169(12), 1284–1291. doi: 10.1176/appi.ajp.2012.12050600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai N, Kawanishi M, Kudomi N, Maeda Y, Yamamoto Y, Nishiyama Y, et al. (2013). Detection of brain amyloid beta deposition in patients with neuropsychological impairment after traumatic brain injury: PET evaluation using Pittsburgh Compound-B. [Research Support, Non-U.S. Gov’t]. Brain Injury, 27(9), 1026–1031. doi: 10.3109/02699052.2013.794963. [DOI] [PubMed] [Google Scholar]
- Kim J, Whyte J, Patel S, Europa E, Slattery J, Coslett HB, et al. (2012a). A perfusion fMRI study of the neural correlates of sustained-attention and working-memory deficits in chronic traumatic brain injury. [Comparative Study Research Support, N.I.H., Extramural]. Neurorehabilitation and Neural Repair, 26(7), 870–880. doi: 10.1177/1545968311434553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Whyte J, Patel S, Europa E, Wang J, Coslett HB, et al. (2012b). Methylphenidate modulates sustained attention and cortical activation in survivors of traumatic brain injury: a perfusion fMRI study. [Randomized Controlled Trial Research Support, N.I.H., Extramural]. Psychopharmacology, 222(1), 47–57. doi: 10.1007/s00213-011-2622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Branch CA, Kim M, & Lipton ML (2013). Whole brain approaches for identification of microstructural abnormalities in individual patients: comparison of techniques applied to mild traumatic brain injury. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. PloS One, 8(3), e59382. doi: 10.1371/journal.pone.0059382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrell T, Leulf C, Cardwell D, Komoroski RA, & Freeman TW (2005). Relationship of in vivo medial temporal lobe magnetic resonance spectroscopy to documented combat exposure in veterans with chronic posttraumatic stress disorder. Psychiatry Research, 140(1), 91–94. doi: 10.1016/j.pscychresns.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kirov I, Fleysher L, Babb JS, Silver JM, Grossman RI, & Gonen O (2007). Characterizing ‘mild’ in traumatic brain injury with proton MR spectroscopy in the thalamus: initial findings. [Research Support, N.I.H., Extramural]. Brain Injury, 21(11), 1147–1154. doi: 10.1080/02699050701630383. [DOI] [PubMed] [Google Scholar]
- Kirov II, Tal A, Babb JS, Lui YW, Grossman RI, & Gonen O (2013a). Diffuse axonal injury in mild traumatic brain injury: a 3D multivoxel proton MR spectroscopy study. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Journal of Neurology, 260(1), 242–252. doi: 10.1007/s00415-012-6626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov II, Tal A, Babb JS, Reaume J, Bushnik T, Ashman TA, et al. (2013b). Proton MR spectroscopy correlates diffuse axonal abnormalities with post-concussive symptoms in mild traumatic brain injury. [Research Support, N.I.H., Extramural]. Journal of Neurotrauma, 30(13), 1200–1204. doi: 10.1089/neu.2012.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte IK, Lin AP, Muehlmann M, Merugumala S, Liao H, Starr T, et al. (2015a). Altered neurochemistry in former professional soccer players without a history of concussion. Journal of Neurotrauma. doi: 10.1089/neu.2014.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte IK, Lin AP, Willems A, Muehlmann M, Hufschmidt J, Coleman MJ, et al. (2015b). A review of neuroimaging findings in repetitive brain trauma. Brain Pathology, 25(3), 318–349. doi: 10.1111/bpa.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaConte SM, Peltier SJ, & Hu XP (2007). Real-time fMRI using brain-state classification. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Human Brain Mapping, 28(10), 1033–1044. doi: 10.1002/hbm.20326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Wilde E, Troyanskaya M, Petersen NJ, Scheibel R, Newsome M, et al. (2010). Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-PH.S.]. Journal of Neurotrauma, 27(4), 683–694. doi: 10.1089/neu.2009.1073. [DOI] [PubMed] [Google Scholar]
- Lewine JD, Davis JT, Sloan JH, Kodituwakku PW, & Orrison WW Jr. (1999). Neuromagnetic assessment of pathophysiologic brain activity induced by minor head trauma. [Comparative Study]. AJNR. American Journal of Neuroradiology, 20(5), 857–866. [PMC free article] [PubMed] [Google Scholar]
- Lewine JD, Davis JT, Bigler ED, Thoma R, Hill D, Funke M, et al. (2007). Objective documentation of traumatic brain injury subsequent to mild head trauma: multimodal brain imaging with MEG, SPECT, and MRI. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.]. The Journal of Head Trauma Rehabilitation, 22(3), 141–155. doi: 10.1097/01.HTR.0000271115.29954.27. [DOI] [PubMed] [Google Scholar]
- Lin A, Ramadan S, Box H, Stanwell P, & Stern RA (2010). Neurochemical Changes in Athletes with Chronic Traumatic Encephalopathy. Paper presented at the 96th Scientific Assembly and Annual Meeting of the Radiological Society of North America, Chicago, IL. [Google Scholar]
- Lin A, Tran T, Bluml S, Merugumala S, Liao HJ, & Ross BD (2012a). Guidelines for acquiring and reporting clinical neurospectroscopy. [Review]. Seminars in Neurology, 32(4), 432–453. doi: 10.1055/s-0032-1331814. [DOI] [PubMed] [Google Scholar]
- Lin AP, Liao HJ, Merugumala SK, Prabhu SP, Meehan WP 3rd, & Ross BD (2012b). Metabolic imaging of mild traumatic brain injury. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non- P.H.S. Review]. Brain Imaging and Behavior, 6(2), 208–223. doi: 10.1007/s11682-012-9181-4. [DOI] [PubMed] [Google Scholar]
- Lin Y, Daducci A, Meskaldji DE, Thiran J, Michel P, Meuli R, et al. (2014). Quantitative analysis of myelin and axonal remodeling in the uninjured motor network after stroke. Brain Connectivity, doi: 10.1089/brain.2014.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AP, Ramadan S, Stern RA, Box HC, Nowinski CJ, Ross D, et al. (2015). Changes in the neurochemistry of athletes with repetitive brain trauma: preliminary results using localized correlated spectroscopy. Alzheimer’s Research & Therapy, 7(1), 13. doi: 10.1186/s13195-015-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling G, Bandak F, Armonda R, Grant G, & Ecklund J (2009). Explosive blast neurotrauma. [Review]. Journal of Neurotrauma, 26(6), 815–825. doi: 10.1089/neu.2007.0484. [DOI] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, & Tannock R (2000). Executive control problems in childhood psychopathology: Stop-signal studies of attention deficit disorder In Monsell S (Ed.), Attention and performance XVIII (pp. 653–677). Cambridge, MA: MIT Press. [Google Scholar]
- Lopez-Larson M, King JB, McGlade E, Bueler E, Stoeckel A, Epstein DJ, et al. (2013). Enlarged thalamic volumes and increased fractional anisotropy in the thalamic radiations in veterans with suicide behaviors. Frontiers in Psychiatry, 4, 83. doi: 10.3389/fpsyt.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Xu D, Roskos T, Stout J, Kull L, Cheng X, et al. (2013). Complexity analysis ofresting state magnetoencephalography activity in traumatic brain injury patients. [Research Support, U.S. Gov’t, Non-P.H.S.]. Journal of Neurotrauma, 30(20), 1702–1709. doi: 10.1089/neu.2012.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, et al. (2011). Detection of blast-related traumatic brain injury in U.S. military personnel. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.]. The New England Journal of Medicine, 364(22), 2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald C, Johnson A, Cooper D, Malone T, Sorrell J, Shimony J, et al. (2013). Cerebellar white matter abnormalities following primary blast injury in US military personnel. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non- P.H.S.]. PloS One, 8(2), e55823. doi: 10.1371/journal.pone.0055823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maikusa N, Yamashita F, Tanaka K, Abe O, Kawaguchi A, Kabasawa H, et al. (2013). Improved volumetric measurement of brain structure with a distortion correction procedure using an ADNI phantom. [Research Support, Non-U.S. Gov’t]. Medical Physics, 40(6), 062303. doi: 10.1118/1.4801913. [DOI] [PubMed] [Google Scholar]
- Makoroff KL, Cecil KM, Care M, & Ball WS Jr. (2005). Elevated lactate as an early marker of brain injury in inflicted traumatic brain injury. Pediatric Radiology, 35(7), 668–676. doi: 10.1007/s00247-005-1441-7. [DOI] [PubMed] [Google Scholar]
- Maksimovskiy AL, McGlinchey RE, Fortier CB, Salat DH, Milberg WP, & Oscar-Berman M (2014). White matter and cognitive changes in veterans diagnosed with alcoholism and PTSD. Journal of Alcoholism & Drug Dependence, 2(1), 144. doi: 10.4172/2329-6488.1000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamere AE, Saraiva LA, Matos AL, Carneiro AA, & Santos AC (2009). Evaluation of delayed neuronal and axonal damage secondary to moderate and severe traumatic brain injury using quantitative MR imaging techniques. [Evaluation Studies]. AJNR. American Journal of Neuroradiology, 30(5), 947–952. doi: 10.3174/ajnr.A1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino S, Ciurleo R, Bramanti P, Federico A, & De Stefano N (2011). 1H-MR spectroscopy in traumatic brain injury. [Review]. Neurocritical Care, 14(1), 127–133. doi: 10.1007/s12028-010-9406-6. [DOI] [PubMed] [Google Scholar]
- Marquez de la Plata C, Ardelean A, Koovakkattu D, Srinivasan P, Miller A, Phuong V, et al. (2007). Magnetic resonance imaging of diffuse axonal injury: quantitative assessment of white matter lesion volume. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.]. Journal of Neurotrauma, 24(4), 591–598. doi: 10.1089/neu.2006.0214. [DOI] [PubMed] [Google Scholar]
- Mascalchi M, Toschi N, Ginestroni A, Giannelli M, Nicolai E, Aiello M, et al. (2014). Gender, age-related, and regional differences of the magnetization transfer ratio of the cortical and subcortical brain gray matter. [Research Support, Non-U.S. Gov’t]. Journal of Magnetic Resonance Imaging, 40(2), 360–366. doi: 10.1002/jmri.24355. [DOI] [PubMed] [Google Scholar]
- Matthews S, Simmons A, & Strigo I (2011a). The effects of loss versus alteration of consciousness on inhibition-related brain activity among individuals with a history of blast-related concussion. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.]. Psychiatry Research, 191(1), 76–79. doi: 10.1016/j.pscychresns.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Strigo IA, Simmons AN, O’Connell RM, Reinhardt LE, & Moseley SA (2011b). A multimodal imaging study in U.S. veterans of Operations Iraqi and Enduring Freedom with and without major depression after blast-related concussion. [Research Support, U.S. Gov’t, Non-P.H.S.]. NeuroImage, 54(Suppl 1), S69–S75. doi: 10.1016/j.neuroimage.2010.04.269. [DOI] [PubMed] [Google Scholar]
- Maugans TA, Farley C, Altaye M, Leach J, & Cecil KM (2012). Pediatric sports-related concussion produces cerebral blood flow alterations. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Pediatrics, 129(1), 28–37. doi: 10.1542/peds.2011-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Gasparovic C, & Yeo RA(2011). Functional connectivity in mild traumatic brain injury. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.]. Human Brain Mapping, 32(11), 1825–1835. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Bedrick EJ, Ling JM, Toulouse T, & Dodd A (2014). Methods for identifying subject-specific abnormalities in neuroimaging data. Human Brain Mapping, 35(11), 5457–5470. doi: 10.1002/hbm.22563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan JC, Yang JH, Plotkin RC, Grossman RI, Umile EM, Cecil KM, et al. (2000). Magnetization transfer imaging in the detection of injury associated with mild head trauma. [Comparative Study Research Support, U.S. Gov’t, P.H.S.]. AJNR. American Journal of Neuroradiology, 21(5), 875–880. [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, Owens EM, RezaBerenji G, Peppers DC, Liang LJ, & Licht EA (2013). Mild traumatic brain injury from primary blast vs. blunt forces: post-concussion consequences and functional neuroimaging. [Research Support, U.S. Gov’t, Non-P.H.S.]. NeuroRehabilitation, 32(2), 397–407. doi: 10.3233/NRE-130861. [DOI] [PubMed] [Google Scholar]
- Mitsis EM, Riggio S, Kostakoglu L, Dickstein DL, Machac J, Delman B, et al. (2014). Tauopathy PET and amyloid PET in the diagnosis of chronic traumatic encephalopathies: studies of a retired NFL player and of a man with FTD and a severe head injury. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.]. Translational Psychiatry, 4, e441. doi: 10.1038/tp.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett JR, Arun P, Ariyannur PS, & Namboodiri AM (2013). N-Acetylaspartate reductions in brain injury: impact on post-injury neuroenergetics, lipid synthesis, and protein acetylation. [Review]. Frontiers in Neuroenergetics, 5, 11. doi: 10.3389/fnene.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Haswell CC, Selgrade ES, Massoglia D, Liu C, Weiner J, et al. (2013). Effects of chronic mild traumatic brain injury on white matter integrity in Iraq and Afghanistan war veterans. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.]. Human Brain Mapping, 34(11), 2986–2999. doi: 10.1002/hbm.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsaerts HJ, Steketee RM, Heijtel DF, Kuijer JP, van Osch MJ, Majoie CB, et al. (2014). Inter-vendor reproducibility of pseudo-continuous arterial spin labeling at 3 Tesla. [Research Support, Non-U.S. Gov’t]. PloS One, 9(8), e104108. doi: 10.1371/journal.pone.0104108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayana PA, Yu X, Hasan KM, Wilde EA, Levin HS, Hunter JV, et al. (2015). Multi-modal MRI of mild traumatic brain injury.[Research Support, U.S. Gov’t, Non-P.H.S.]. NeuroImage: Clinical,87–97. doi: 10.1016/j.nicl.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DE, Oakes TR, Yeh PH, French LM, Harper JF, Liu W, et al. (2015). Exploring variations in functional connectivity of the resting state default mode network in mild traumatic brain injury. Brain Connectivity, 5(2), 102–114. doi: 10.1089/brain.2014.0273. [DOI] [PubMed] [Google Scholar]
- Newbould RD, Nicholas R, Thomas CL, Quest R, Lee JS, Honeyfield L, et al. (2014). Age independently affects myelin integrity as detected by magnetization transfer magnetic resonance imaging in multiple sclerosis. NeuroImage: Clinical, 4, 641–648. doi: 10.1016/j.nicl.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome MR, Durgerian S, Mourany L, Scheibel RS, Lowe MJ, Beall EB, et al. (2015). Disruption of caudate working memory activation in chronic blast-related traumatic brain injury. NeuroImage: Clinical, 8, 543–553. doi: 10.1016/j.nicl.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TS, Lin AP, Koerte IK, Pasternak O, Liao H, Merugumala S, et al. (2014). Neuroimaging in repetitive brain trauma. [Review]. Alzheimer’s Research & Therapy, 6(1), 10. doi: 10.1186/alzrt239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer E (2005). Electroencephalography: Basic principles, clinical applications, and related fields (5th ed.). Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, et al. (1992). Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. Proceedings of the National Academy of Sciences of the United States of America, 89(13), 5951–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, et al. . Clinical proton MR spectroscopy in central nervous system disorders. [Research Support, Non-U.S. Gov’t Review]. Radiology, 270(3), 658–679. doi: 10.1148/radiol.13130531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani E, Bizzi A, Di Salle F, De Stefano N, & Filippi M (2008). Basic concepts of advanced MRI techniques. [Review]. Neurological Sciences, 29(Suppl 3), 290–295. doi: 10.1007/s10072-008-1001-7. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Sochen N, Gur Y, Intrator N, & Assaf Y (2009). Free water elimination and mapping from diffusion MRI. [Research Support, Non-U.S. Gov’t]. Magnetic Resonance in Medicine, 62(3), 717–730. doi: 10.1002/mrm.22055. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Petrie EC, Cross DJ, Pagulayan K, McCraw K, Hoff D, et al. (2011). Cerebrocerebellarhypometabolismassociat-ed with repetitive blast exposure mild traumatic brain injury in 12 Iraq war Veterans with persistent post-concussive symptoms. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.]. NeuroImage, 54(Suppl 1), S76–S82. doi: 10.1016/j.neuroimage.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie EC, Cross DJ, Yarnykh VL , Richards T, Martin NM, Pagulayan K, et al. (2014). Neuroimaging, behavioral, andpsycho-logical sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.]. Journal of Neurotrauma, 31(5), 425–436. doi: 10.1089/neu.2013.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole VN , Abbas K, Shenk TE, Breedlove EL, Breedlove KM, Robinson ME, et al. (2014). MR spectroscopic evidence of brain injury in the non-diagnosed collision sport athlete. [Observational Study Research Support, Non-U.S. Gov’t]. Developmental Neuropsychology, 39(6), 459–473. doi: 10.1080/87565641.2014.940619. [DOI] [PubMed] [Google Scholar]
- Poole VN, Breedlove EL, Shenk TE, Abbas K, Robinson ME, Leverenz LJ, et al. (2015). Sub-concussive hit characteristics predict deviant brain metabolism in football athletes. [Research Support, Non-U.S. Gov’t]. Developmental Neuropsychology, 40(1), 12–17. doi: 10.1080/87565641.2014.984810. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard A, & Shulman GL (2001). A default mode of brain function. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, et al. (2011). Inflammation after trauma: microglial activation and traumatic brain injury. [Research Support, Non-U.S. Gov’t]. Annals of Neurology, 70(3), 374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- Raymont V, Salazar AM, Lipsky R, Goldman D, Tasick G, & Grafman J (2010). Correlates of posttraumatic epilepsy 35 years following combat brain injury. [Research Support, N.I.H., Intramural Research Support, U.S. Gov’t, Non-P.H.S.]. Neurology, 75(3), 224–229. doi: 10.1212/WNL.0b013e3181e8e6d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reider G II, Groswasser Z, Ommaya AK, Schwab K, Pridgen A, Brown HR, et al. (2002). Quantitive imaging in late traumatic brain injury. Part I: late imaging parameters in closed and penetrating head injuries. [Clinical Trial]. Brain Injury, 16(6), 517–525. doi: 10.1080/02699050110119141. [DOI] [PubMed] [Google Scholar]
- Robinson ME, Lindemer ER, Fonda JR, Milberg WP, McGlinchey RE, & Salat DH (2015). Close-range blast exposure is associated with altered functional connectivity in Veterans independent of concussion symptoms at time of exposure. [Research Support, U.S. Gov’t, Non-P.H.S.]. Human Brain Mapping, 36(3), 911–922. doi: 10.1002/hbm.22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross BD, Ernst T, Kreis R, Haseler LJ, Bayer S, Danielsen E, et al. (1998). 1H MRS in acute traumatic brain injury. [Research Support, Non-U.S. Gov’t]. Journal of Magnetic Resonance Imaging, 8(4), 829–840. [DOI] [PubMed] [Google Scholar]
- Rubovitch V, Ten-Bosch M, Zohar O, Harrison CR, Tempel-Brami C, Stein E, et al. (2011). A mouse model of blast-induced mild traumatic brain injury. [Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.]. Experimental Neurology, 232(2), 280–289. doi: 10.1016/j.expneurol.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthotto L, Kugel H, Olesch J, Fischer B, Modersitzki J, Burger M, et al. (2012). Diffeomorphic susceptibility artifact correction of diffusion-weighted magnetic resonance images. [Research Support, Non-U.S. Gov’t]. Physics in Medicine and Biology, 57(18), 5715–5731. doi: 10.1088/0031-9155/57/18/5715. [DOI] [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Steinberg JL, Pearson DA, Rauch RA, Mao H, et al. (2007). Altered brain activation during cognitive control in patients with moderate to severe traumatic brain injury. [Research Support, N.I.H., Extramural]. Neurorehabilitation and Neural Repair, 21(1), 36–45. doi: 10.1177/1545968306294730. [DOI] [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Troyanskaya M, Steinberg JL, Goldstein FC, Mao H, et al. (2009). Effects of severity of traumatic brain injury and brain reserve on cognitive-control related brain activation. [Research Support, N.I.H., Extramural]. Journal of Neurotrauma, 26(9), 1447–1461. doi: 10.1089/neu.2008.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Troyanskaya M, Lin X, Steinberg JL, Radaideh M, et al. (2012). Altered brain activation in military personnel with one or more traumatic brain injuries following blast. [Research Support, U.S. Gov’t, Non-P.H.S.]. Journal of the International Neuropsychological Society, 18(1), 89–100. doi: 10.1017/S1355617711001433. [DOI] [PubMed] [Google Scholar]
- Schuff N, Neylan TC, Fox-Bosetti S, Lenoci M, Samuelson KW, Studholme C, et al. (2008). Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. [Research Support, U.S. Gov’t, Non-P.H.S.]. Psychiatry Research, 162(2), 147–157. doi: 10.1016/j.pscychresns.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selwyn R, Hockenbury N, Jaiswal S, Mathur S, Armstrong RC, & Byrnes KR (2013). Mild traumatic brain injury results in depressed cerebral glucose uptake: an (18)FDG PET study. [Research Support, Non-U.S. Gov’t]. Journal of Neurotrauma, 30(23), 1943–1953. doi: 10.1089/neu.2013.2928. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, et al. (2012). A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review]. Brain Imaging and Behavior, 6(2), 137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutter L, Tong KA, & Holshouser BA (2004). Proton MRS in acute traumatic brain injury: role for glutamate/glutamine and choline for outcome prediction. [Research Support, Non-U.S. Gov’t]. Journal of Neurotrauma, 21(12), 1693–1705. doi: 10.1089/neu.2004.21.1693. [DOI] [PubMed] [Google Scholar]
- Signoretti S, Marmarou A, Tavazzi B, Lazzarino G, Beaumont A, & Vagnozzi R (2001). N-Acetylaspartate reduction as a measure of injury severity and mitochondrial dysfunction following diffuse traumatic brain injury. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. Journal of Neurotrauma, 18(10), 977–991. doi: 10.1089/08977150152693683. [DOI] [PubMed] [Google Scholar]
- Sinson G, Bagley LJ, Cecil KM, Torchia M, McGowan JC, Lenkinski RE, et al. (2001). Magnetization transfer imaging and proton MR spectroscopy in the evaluation ofaxonal injury: correlation with clinical outcome after traumatic brain injury. [Research Support, U.S. Gov’t, P.H.S.]. AJNR. American Journal of Neuroradiology, 22(1), 143–151. [PMC free article] [PubMed] [Google Scholar]
- Sokunbi MO, Linden DE, Habes I, Johnston S, & Ihssen N (2014). Real-time fMRI brain-computer interface: development of a “motivational feedback” subsystem for the regulation of visual cue reactivity. Frontiers in Behavioral Neuroscience, 8, 392. doi: 10.3389/fnbeh.2014.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, & Cross AH (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. NeuroImage, 17(3), 1429–1436. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, & Neufeld A (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. NeuroImage, 20(3), 1714–1722. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, et al. (2005). Demyelination increases radial diffusivity in corpus callosum of mouse brain. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. NeuroImage, 26(1), 132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Spielberg JM, McGlinchey RE, Milberg WP, & Salat DH(2015). Brain network disturbance related to posttraumatic stress and traumatic brain injury in veterans. Biological Psychiatry, 78(3), 210–216. doi: 10.1016/j.biopsych.2015.02.013. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, McGuire KA, Kang SS, Davenport ND, Aviyente S, Bernat EM, et al. (2011). Evidence of disrupted functional connectivity in the brain after combat-related blast injury. [Research Support, U.S. Gov’t, Non-P.H.S.]. NeuroImage, 54(Suppl 1), S21–S29. doi: 10.1016/j.neuroimage.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Sporns O, Chialvo DR, Kaiser M, & Hilgetag CC (2004). Organization, development and function of complex brain networks. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-PH.S. Research Support, U.S. Gov’t, P.H.S. Review]. Trends in Cognitive Sciences, 8(9), 418–425. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Sternberg S (1966). High-speed scanning in human memory. Science, 153(3736), 652–654. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Lovejoy D, Kim J, Oakes H, Kureshi I, & Witt ST (2012). Multiple resting state network functional connectivity abnormalities in mild traumatic brain injury. [Research Support, Non-U.S. Gov’t]. Brain imaging and Behavior, 6(2), 293–318. doi: 10.1007/s11682-012-9157-4. [DOI] [PubMed] [Google Scholar]
- Stocker RP, Cieply MA, Paul B, Khan H, Henry L, Kontos AP, et al. (2014). Combat-related blast exposure and traumatic brain injury influence brain glucose metabolism during REM sleep in military veterans. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.]. Neuroimage, 99, 207–214. doi: 10.1016/j.neuroimage.2014.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symms M, Jager HR, Schmierer K, & Yousry TA (2004). A review of structural magnetic resonance neuroimaging. [Review]. Journal ofNeurology, Neurosurgery, and Psychiatry, 75(9), 1235–1244. doi: 10.1136/jnnp.2003.032714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber KH, Hurley RA, Haswell CC, Rowland JA, Hurt SD, Lamar CD, et al. (2015). White matter compromise in veterans exposed to primary blast forces. [Research Support, U.S. Gov’t, Non-P.H.S.]. The Journal of Head Trauma Rehabilitation, 30(1), E15–E25. doi: 10.1097/HTR.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarapore PE, Findlay AM, Lahue SC, Lee H, Honma SM, Mizuiri D, et al. (2013). Resting state magnetoencephalography functional connectivity in traumatic brain injury. [Case Reports Research Support, N.I.H., Extramural]. Journal of Neurosurgery, 118(6), 1306–1316. doi:10.317½013.3.JNS12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate DF, York GE, Reid MW, Cooper DB, Jones L, Robin DA, et al. (2014). Preliminary findings of cortical thickness abnormalities in blast injured service members and their relationship to clinical findings. [Research Support, Non-U.S. Gov’t]. Brain imaging and Behavior, 8(1), 102–109. doi: 10.1007/s11682-013-9257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins P, Tesiram Y, Lerner M, Gonzalez LP, Lightfoot S, Rabb CH, et al. (2013). Brain injury: neuro-inflammation, cognitive deficit, and magnetic resonance imaging in a model of blast induced traumatic brain injury. Journal of Neurotrauma, 30(22), 1888–1897. doi: 10.1089/neu.2012.2674. [DOI] [PubMed] [Google Scholar]
- Tong KA, Ashwal S, Holshouser BA, Shutter LA, Herigault G, Haacke EM, et al. (2003). Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. [Comparative Study]. Radiology, 227(2), 332–339. doi: 10.1148/radiol.2272020176. [DOI] [PubMed] [Google Scholar]
- Tormenti M, Krieger D, Puccio AM, McNeil MR, Schneider W, & Okonkwo DO (2012). Magnetoencephalographic virtual recording: a novel diagnostic tool for concussion. [Controlled Clinical Trial Research Support, Non-U.S. Gov’t Validation Studies]. Neurosurgical Focus, 33(6), E9. doi:10.317½012.10.FOCUS12282. 1–7. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R, Signoretti S, Tavazzi B, Floris R, Ludovici A, Marziali S, et al. (2008). Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athletes-part III. [Controlled Clinical Trial]. Neurosurgery, 62(6), 1286–1295. doi: 10.1227/01.neu.0000333300.34189.74. discussion 1295–1286. [DOI] [PubMed] [Google Scholar]
- Vakhtin AA, Calhoun VD, Jung RE, Prestopnik JL, Taylor PA, & Ford CC (2013). Changes in intrinsic functional brain networks following blast-induced mild traumatic brain injury. [Research Support, U.S. Gov’t, Non-P.H.S.]. Brain injury, 27(11), 1304–1310. doi: 10.3109/02699052.2013.823561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z (2014). Support vector machine learning-based cerebral blood flow quantification for arterial spin labeling MRI. [Research Support, N.I.H., Extramural]. Human Brain Mapping, 35(7), 2869–2875. doi: 10.1002/hbm.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DJ, Alger JR, Qiao JX, Gunther M, Pope WB, Saver JL, et al. (2013). Multi-delay multi-parametric arterial spin-labeled perfusion MRI in acute ischemic stroke - comparison with dynamic susceptibility contrast enhanced perfusion imaging. Neuroimage: Clinical, 3, 1–7. doi: 10.1016/j.nicl.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cusick MF, Wang Y, Sun P, Libbey JE, Trinkaus K, et al. (2014). Diffusion basis spectrum imaging detects and distinguishes coexisting subclinical inflammation, demyelination and axonal injury in experimental autoimmune encephalomyelitis mice. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.]. NMR in Biomedicine, 27(7), 843–852. doi: 10.1002/nbm.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sun P, Wang Q, Trinkaus K, Schmidt RE, Naismith RT, et al. (2015a). Differentiation and quantification of inflammation, demyelination and axon injury or loss in multiple sclerosis. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Brain, 138(Pt 5), 1223–1238. doi: 10.1093/brain/awv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sun P, Wang Q, Trinkaus K, Schmidt RE, Naismith RT, et al. (2015b). Differentiation and quantification of inflammation, demyelination and axon injury or loss in multiple sclerosis. Brain. doi: 10.1093/brain/awv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, West JD, Bailey JN, Westfall DR, Xiao H, Arnold TW, et al. (2015c). Decreased cerebral blood flow in chronic pediatric mild TBI: an MRI perfusion study. [Research Support, N.I.H., Extramural]. Developmental Neuropsychology, 40(1), 40–44. doi: 10.1080/87565641.2014.979927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, et al. (2008). Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Neuroimage, 41(4), 1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Hunter JV, Newsome MR, Scheibel RS, Bigler E , Johnson JL, et al. (2005). Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. Journal of Neurotrauma, 22(3), 333–344. doi: 10.1089/neu.2005.22.333. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Layer V, Gregori J, Griebe M, Szabo K, Gass A, et al. (2014). Assessment of perfusion deficits in ischemic stroke using 3D-GRASE arterial spin labeling magnetic resonance imaging with multiple inflow times. [Research Support, Non-U.S. Gov’t]. Journal of Neuroimaging, 24(5), 453–459. [DOI] [PubMed] [Google Scholar]
- Yeo RA, Gasparovic C, Merideth F, Ruhl D, Doezema D, & Mayer AR (2011). A longitudinal proton magnetic resonance spectroscopy study of mild traumatic brain injury. [Research Support, N.I.H., Extramural]. Journal of Neurotrauma, 28(1), 1–11. doi: 10.1089/neu.2010.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo RE, Yun TJ, Rhim JH, Yoon BW, Kang KM, Choi SH, et al. (2015). Bright vessel appearance on arterial spin labeling MRI for localizing arterial occlusion in acute ischemic stroke. [Research Support, Non-U.S. Gov’t]. Stroke, 46(2), 564–567. doi: 10.1161/STROKEAHA.114.007797. [DOI] [PubMed] [Google Scholar]
- Yuan H, Young KD, Phillips R, Zotev V, Misaki M, & Bodurka J (2014). Resting-state functional connectivity modulation and sustained changes after real-time functional magnetic resonance imaging neurofeedback training in depression. [Research Support, Non-U.S. Gov’t]. Brain Connectivity, 4(9), 690–701. doi: 10.1089/brain.2014.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Bueler CE, McGlade EC, Churchwell JC, Brenner LA, & Lopez-Larson MP (2011). Neuroimaging correlates of traumatic brain injury and suicidal behavior. [Comparative Study Research Support, Non-U.S. Gov’t]. The Journal of Head Trauma Rehabilitation, 26(4), 276–289. doi: 10.1097/HTR.0b013e31822251dc. [DOI] [PubMed] [Google Scholar]
- Zaharchuk G, Straka M, Marks MP, Albers GW, Moseley ME, & Bammer R (2010). Combined arterial spin label and dynamic susceptibility contrast measurement of cerebral blood flow. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Magnetic Resonance in Medicine, 63(6), 1548–1556. doi: 10.1002/mrm.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, & Li CS (2012). Functional networks for cognitive control in a stop signal task: independent component analysis. [Research Support, N.I.H., Extramural]. Human Brain Mapping, 33(1), 89–104. doi: 10.1002/hbm.21197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Mitsis EM, Chu K, Newmark RE, Hazlett EA, & Buchsbaum MS (2010). Statistical parametric mapping and cluster counting analysis of [18F] FDG-PET imaging in traumatic brain injury. Journal of Neurotrauma, 27(1), 35–49. doi: 10.1089/neu.2009.1049. [DOI] [PubMed] [Google Scholar]
- Zhang H, Schneider T, Wheeler-Kingshott CA, & Alexander DC (2012). NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. [Research Support, Non-U.S. Gov’t]. NeuroImage, 61(4), 1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
- Zhang K, Herzog H, Mauler J, Filss C, Okell TW, Kops ER, et al. (2014). Comparison of cerebral blood flow acquired by simultaneous [15O]water positron emission tomography and arterial spin labeling magnetic resonance imaging. [Comparative Study Research Support, Non-U.S. Gov’t]. Journal of Cerebral Blood Flow and Metabolism, 34(8), 1373–1380. doi: 10.1038/jcbfm.2014.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R, Dwyer MG, Markovic-Plese S, Kennedy C, Bergsland N, Ramasamy DP, et al. (2014). Effect of treatment with interferon beta-1a on changes in voxel-wise magnetization transfer ratio in normal appearing brain tissue and lesions of patients with relapsing-remitting multiple sclerosis: a 24-week, controlled pilot study. [Research Support, Non-U.S. Gov’t]. PloS One, 9(3), e91098. doi: 10.1371/journal.pone.0091098. [DOI] [PMC free article] [PubMed] [Google Scholar]


