Figure 2.
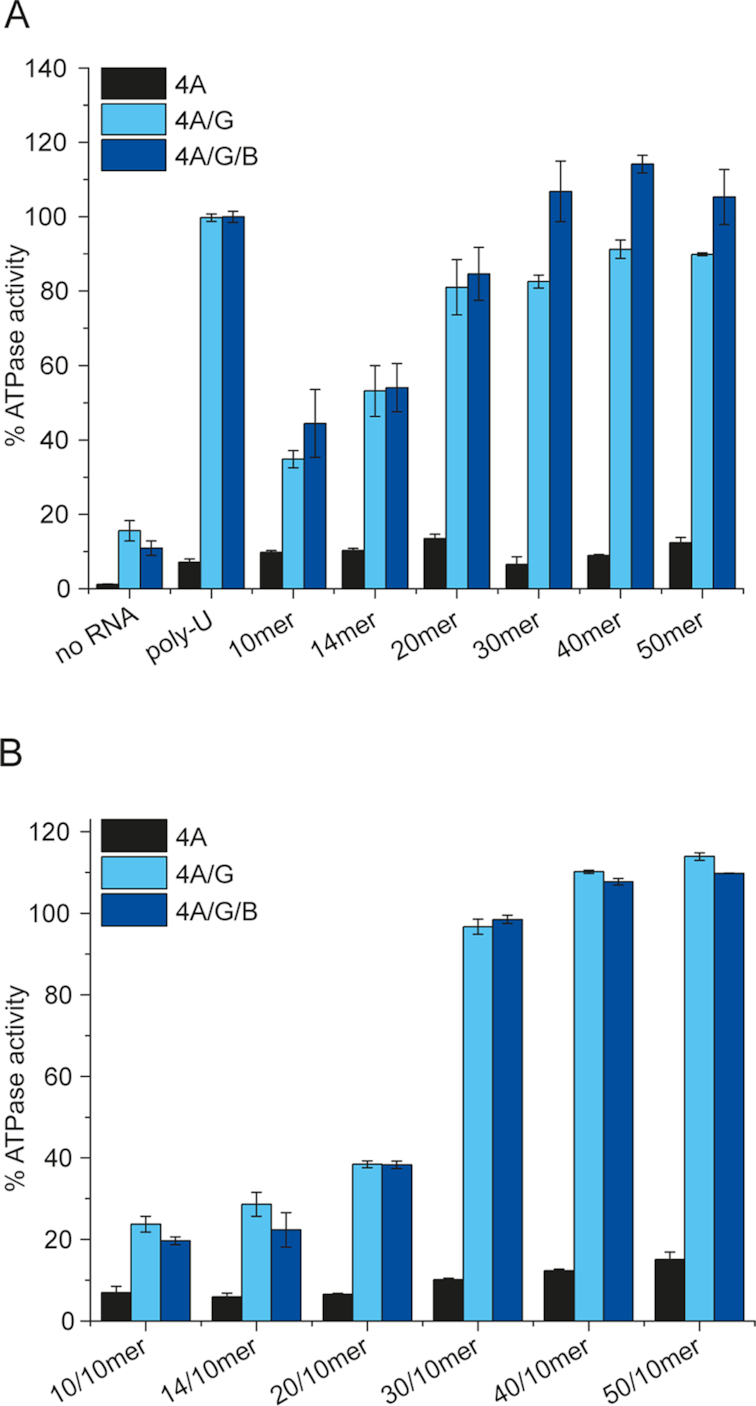
Dependence of the eIF4A steady-state ATPase activity on the length of the RNA substrate and of single-stranded 5′-tails. (A) Rate constant of ATP hydrolysis as a function of length of single-stranded RNA, relative to the ATP hydrolysis in the presence of poly-U RNA (set to 100%, typical values for the rate of ATP hydrolysis in the presence of poly-U RNA range between 70 and 100 × 10−3 s−1). Experiments were performed with 1 μM eIF4A, 2 μM eIF4B and eIF4G, 15 μM RNA (molecular concentration), 2 mM ATP in 30 mM HEPES/KOH, pH 7.4, 100 mM KOAc, 3 mM Mg(OAc)2, 2 mM DTT at 25°C. (B) Rate constant of ATP hydrolysis as a function of length of the 5′-single-stranded region flanking a 10 bp duplex. Black: eIF4A, light blue: eIF4A/eIF4G, dark blue: eIF4A, eIF4B, and eIF4G. Rate constants are given relative to the turnover number in the presence of poly-U RNA (see panel A), which was set to 100%. Experiments were performed at least twice; error bars reflect the standard error of the mean (n = 2) or the standard deviation (n > 2).
