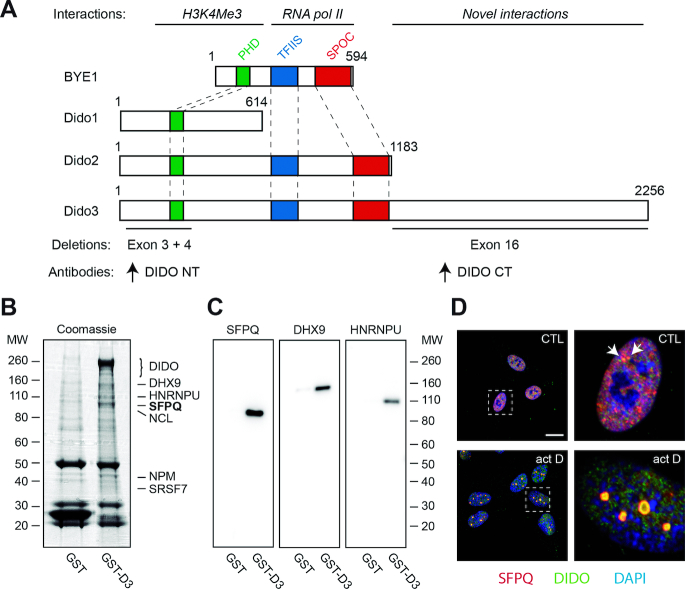
Figure 1.
Interaction between Dido3 and splicing factors. (A) Scheme of Saccharomyces cerevisae BYE1 and Mus musculus Dido proteins, indicating protein interactions (above) and deletions used in this study (below). Colored areas indicate known domain structures. Proteins are drawn to scale. (B) Pull-down experiments using glutathione-S-transferase fused to Dido3. GST and GST-D3 were purified from HEK293T cells and analyzed by SDS-PAGE and Coomassie staining. Interacting proteins identified by proteomics analysis are indicated on the right. (C) WB with indicated antibodies confirmed co-purification. Molecular weight of markers is indicated in kDa (left in B; right in C). (D) RPE-1 cells were seeded on glass coverslips, treated with vehicle only (top) or actinomycin D (bottom), and analyzed by immunofluorescence. Boxed areas show magnification of single nuclei; scale bar: 10 μm.