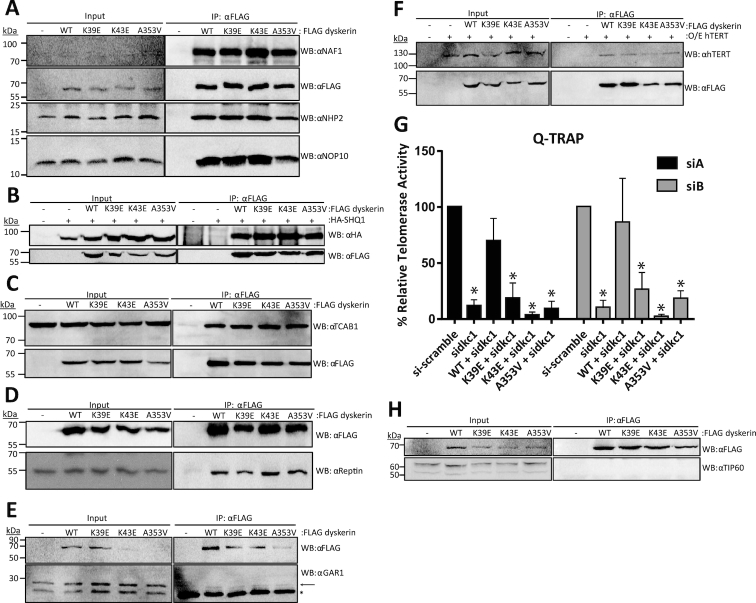
Figure 2.
Dyskerin variants co-immunoprecipitate (co-IP) H/ACA RNP assembly factors comparable to wildtype dyskerin, but result in reduced telomerase activity in cells depleted of endogenous dyskerin. Interactions of FLAG-dyskerin WT and variants with factors needed for assembly of the H/ACA ribonucleoprotein complex and telomerase were assessed by co-immunoprecipitation from HEK293 cell lysates. Assembly of the (A) H/ACA pre-RNP complex involving NAF1 (input protein not detectable), NHP2, and NOP10 was investigated by immunoblotting for the endogenous H/ACA pre-RNP components and FLAG-dyskerin proteins. Interaction with the cytosolic chaperone and RNA mimic (B) SHQ1 was assessed by co-expressing HA-tagged SHQ1 and FLAG-tagged dyskerin, and immunoblotting for HA and FLAG. Similar to the H/ACA pre-RNP complex, the interaction between dyskerin and nuclear RNP assembly factors (C) TCAB1 and (D) reptin and mature H/ACA complex component (E). GAR1 was examined by immunoblotting for endogenous assembly factors and FLAG-dyskerin. (F) The interaction between dyskerin and the telomerase reverse transcriptase hTERT was assessed by co-expressing hTERT and FLAG-tagged dyskerin, and immunoblotting for hTERT and FLAG. (G) Q-TRAP was repeated in experimental replicate n = 3, and quality of telomeric repeat amplification products were visually assessed on 10% non-denaturing acrylamide gel (see Supplementary Figure S1B for representative image). Statistically significant reductions in relative telomerase activity are indicated by * (P value < 0.01). Error bars represent SEM. (H) The nuclear chromatin-associated histone acetyltransferase TIP60 could not be observed in the FLAG-dyskerin IP fraction, though the expected 55 kDa protein band was observed in the input fractions (note that the upper band in the input panel represents a non-specific band that is expected based on the antibody datasheet). Immunoblotting targets are indicated to the right of each panel as ‘WB: α target’, and a list of antibodies can be found in the materials and methods section. In the IP panel of (E) the GAR1-specific band is indicated by an arrow, while immunoglobulin light chain is the strong band present in all IP fractions indicated by the asterisk. Each co-IP and immunoblotting was performed in experimental replicate a minimum of n = 2, representative blots are shown.