Figure 5.
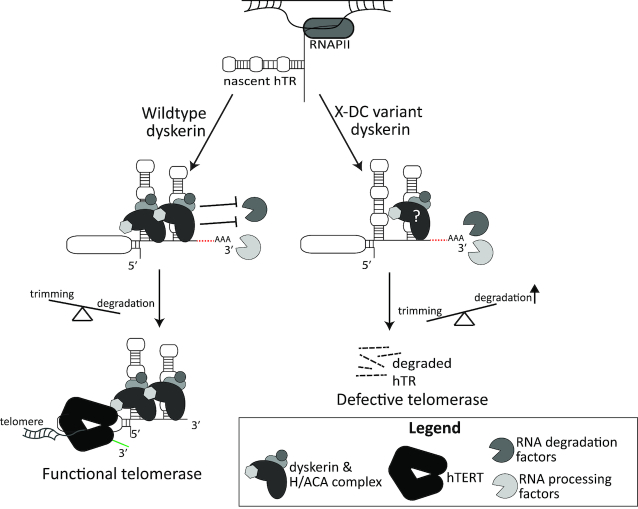
Summary model. Under wildtype conditions, dyskerin binds to newly transcribed hTR, protecting species with 3′ extensions and/or poly(A) tails from degradation. This ensures the trimming of hTR 3′ extended and/or polyadenylated species into mature hTR can take place, followed by assembly of the functional telomerase complex, and telomere maintenance by hTERT. In the context of X-DC, dyskerin variants with defects in binding to newly transcribed hTR result in deprotection of the H/ACA box of hTR, leading to misregulated degradation of 3′ extended and polyadenylated species by a variety of reported RNA processing and degradation complexes (i.e. PABPN1/PARN, NEXT/TRAMP/exosome, DCP2/XRN1, and TOE1). Ultimately, this causes a reduced amount of functional telomerase and a lack of telomere maintenance, as has been observed in X-DC patients. Two copies of the H/ACA ribonucleoprotein complex have been reported to be assembled with the H/ACA box of hTR in active telomerase, with the 5′ stem loop dyskerin likely being anchored to the complex via the 3′ stem loop-bound dyskerin, which may be disrupted by amino acid substitutions in the N-terminal extension or DKCLD X-DC hotspot.