Abstract
Cell plasma membranes are a heterogeneous mixture of lipids and membrane proteins. The importance of heterogeneous lipid domains (also called lipid rafts) as a molecular sorting platform has been implicated in many physiological processes. Cell plasma membranes that are detached from the cytoskeletal structure spontaneously phase separate into distinct domains at equilibrium, which show their inherent demixing properties. Recently, researchers have discovered that proteins with strong interprotein interactions also spontaneously phase separate into distinct protein domains, thus enabling the maintenance of many membraneless organelles. Protein phase separation may also take place on the lipid membranes via lipid-anchored proteins, which suggests another potential molecular sorting platform for physiological processes on the cell membrane. When two-phase separation properties coexist physiologically, they may change the resulting phase behavior or serve as independent sorting platforms. In this paper, we used in vitro reconstitution and fluorescence imaging to systematically quantify the phase behavior that arises when proteins with inherent phase separation properties interact with raft mixture lipid membranes. Our observations and simulations show both that the proteins may enhance lipid phase separation and that this is a general property of phase-separating protein systems with a diverse number of components involved. This suggests that we should consider the overall effect of the properties of both membrane-anchored proteins and lipids when interpreting molecular sorting phenomena on the membranes.
Introduction
Cell plasma membrane is a mixture of many lipids and membrane proteins. Cell membranes are not a completely homogeneous mixture, and heterogeneous composition domains or cell membrane phase domains are known as lipid rafts.1,2 Many biological processes that take place on the membranes involve the sorting of membrane proteins. Examples include membrane trafficking3 and signaling processes such as T-cell, B-cell, and Eph-signaling.4−7 Membrane phase domains can work as a sorting platform for proteins on the membranes because different membrane molecules have different partition preferences for domains.8 Cell plasma membrane detachment vesicles have shown very clear binary phase separation, which is temperature-dependent and reversible.9 The observation suggests that the phase separation is a thermodynamically spontaneous process below the transition temperature. A plasma membrane mimicking a lipid-only ternary mixture system also showed a reversible binary mixing transition, suggesting that the process is largely lipid-driven.10 However, this discrete thermodynamic phase transition has never been observed in living cells.11 Similarly, no micrometer scale phase domains have been observed in living cell membranes except for a special case of the yeast vacuole membranes.12 This is because the cell membrane is closely linked to the actin cytoskeletal structure, which may suppress the phase domain formation by continuously applying tension.13,14 Linkage to the skeletal structure also gives different parts of the membrane different physical properties, promoting a localized physical response.15 Considering all of these findings, the current view of lipid rafts is characterized by the heterogeneous and dynamic fluctuation of the lipid membrane composition, driven by inherent thermodynamic phase separation properties that are influenced by active lipid–protein interactions.16
Thermodynamic phase separation is a commonly used molecular sorting principle for living cells, and it is not only limited to the lipid membrane domains.17−19 Cytosolic proteins can also form phase domains. Phase separation or demixing happens when the energetic advantage of intermolecular interaction is greater than the entropic cost of forming relatively ordered domains. At high concentrations, multivalent binding proteins spontaneously form phase-separated domains or liquid droplets.20 Other phase-separating proteins utilize the interaction of intrinsically disordered regions (IDRs), which often involves interaction with RNA molecules.19,21−23 A phase domain can work as a sorting platform because different cargo molecules have different partition preferences for domains.24,25 A domain may also capture small synaptic vesicles.26 Many membraneless organelles such as p-granules, stress granules, processing bodies, Cajal bodies, and nucleoli are maintained via the spontaneous phase separation of proteins,27,28 and maintaining proper phase domains is important for regular physiological functions.
Recent studies have shown that some membrane-anchored protein systems can exhibit two-dimensional (2D) phase separation on lipid membranes that are independent of lipid-membrane-driven phase separation,29−31 which suggests that anchored protein-driven phase separation may be an important principle of membrane sorting. Since we realize that both anchored protein- and lipid-driven phase separations are relevant for molecular sorting on the membrane, it is important to understand how they interact with each other. The way that protein and lipid domains interact with each other on the membranes has never been studied before. Two different types of phase separations, between lipids and proteins, may compete with or assist each other to form collaborative domains. Alternatively, they may be entirely orthogonal to each other, providing a potential platform for higher-order sorting processes than with binary segregation.
In this study, we used an in vitro reconstitution approach to build systems with inherent lipid-driven and anchored protein-driven separation properties in well-controlled experimental conditions (Figure 1). Ternary mixtures of lipid giant unilamellar vesicles (GUVs) with inherent phase separation behavior were generated, and various protein systems with inherent phase separation behavior were purified to interact on the GUV. Fluorescent tags were introduced for confocal fluorescence imaging. Our observations suggest that anchored proteins with inherent phase separation properties can enhance the overall phase separation in the membrane system.
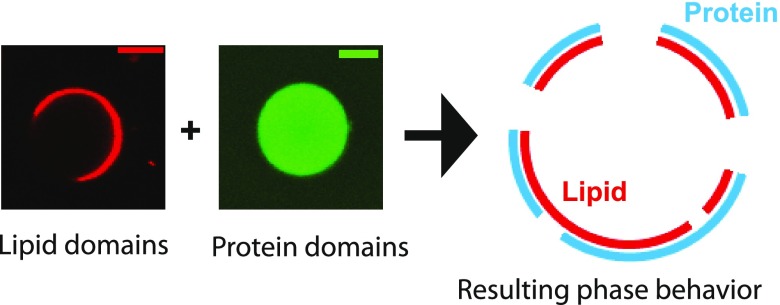
Figure 1.
Reconstitution of interaction between lipid and protein domains. The upper left panel shows a typical confocal fluorescence image of a phase-separated ternary mixture GUV. The signal was from TR-DHPE lipid. The upper right panel shows a typical confocal fluorescence image of a solution phase-separated protein droplet domain from a two-component system (SH3 × 4 and PRM × 4). The signal was from SH3 × 4-Atto488. Scale bars are 5 μm. The lower schematic is a possible outcome of coexistence of both on the lipid bilayer.
Results and Discussion
Two-Component Phase-Separating Proteins Increase the Phase Separation of the Lipid Raft Mixture
We first tried to reconstitute anchored protein-driven phase separation on the lipid membrane environment using a well-known set of proteins with inherent phase separation behavior. SH3 and PRM protein domains, or a two-component system, are an important binding pair in T-cell receptor clustering. T-cell receptor clustering is a part of the T-cell signaling process, which is triggered by antigen recognition.29,31 Multivalent binding between two domains can generate gel-like droplets or phase-separated domains in solution at high concentrations (Figure 1). We anchored four times repeated SH3 domains (SH3 × 4) on the lipid membrane using Ni2+ coordination of the polyhistidine tag to the lipid Ni-NTA and then added 4× repeated PRM domains (PRM × 4) in a solution without the polyhistidine tag. As three-dimensional (3D) protein domains or liquid droplet formation usually happens at a concentration of >100 μM,20 and the lipid environment tends to reduce the necessary concentration,30 we carried out our experiments at protein concentrations of <5 μM.
When we incubated the 2 μM proteins with the control glass-supported lipid bilayer (98% DOPC, 2% Ni-DGS, 0.01% TR-DHPE), we found that the strong interprotein interaction made proteins on the bilayer only partially mobile (Supporting Information, Figure S1). This means that purely protein-driven phase separation of the two-component system that is in fluidic equilibrium on the membrane cannot be achieved at this condition. Next, we studied the effect of interprotein interaction on a ternary mixture lipid GUV with inherent lipid-driven phase separation (Figure 2a). We selected a GUV composition where the vesicles were close to phase separation, but with the majority of vesicles still uniform, or not yet phase-separated (45% DOPC, 20% DPPC, 35% cholesterol, 5% DOPS, 10% Ni-DGS, 0.1% TR-DHPE). This made it easy to quantify how proteins with inherent phase properties affected the membranes.10 A previous study reported GUVs with a similar composition with a higher ratio of phase-separated vesicles than our system.32 This difference could be due to the fact that we had charged lipid species, 5% DOPS, and performed the experiment under the ionic concentration of 100 mM NaCl, which affects the overall phase behavior. As a result, we only interpreted the data within our observations where the condition was controlled in the same way. As negatively charged lipid species are an important major component of the cell plasma membrane,33 we believed that we could better mimic the plasma membrane property by considering the charge interaction. Therefore, we included 5% DOPS in all of our subsequent vesicles in this paper.
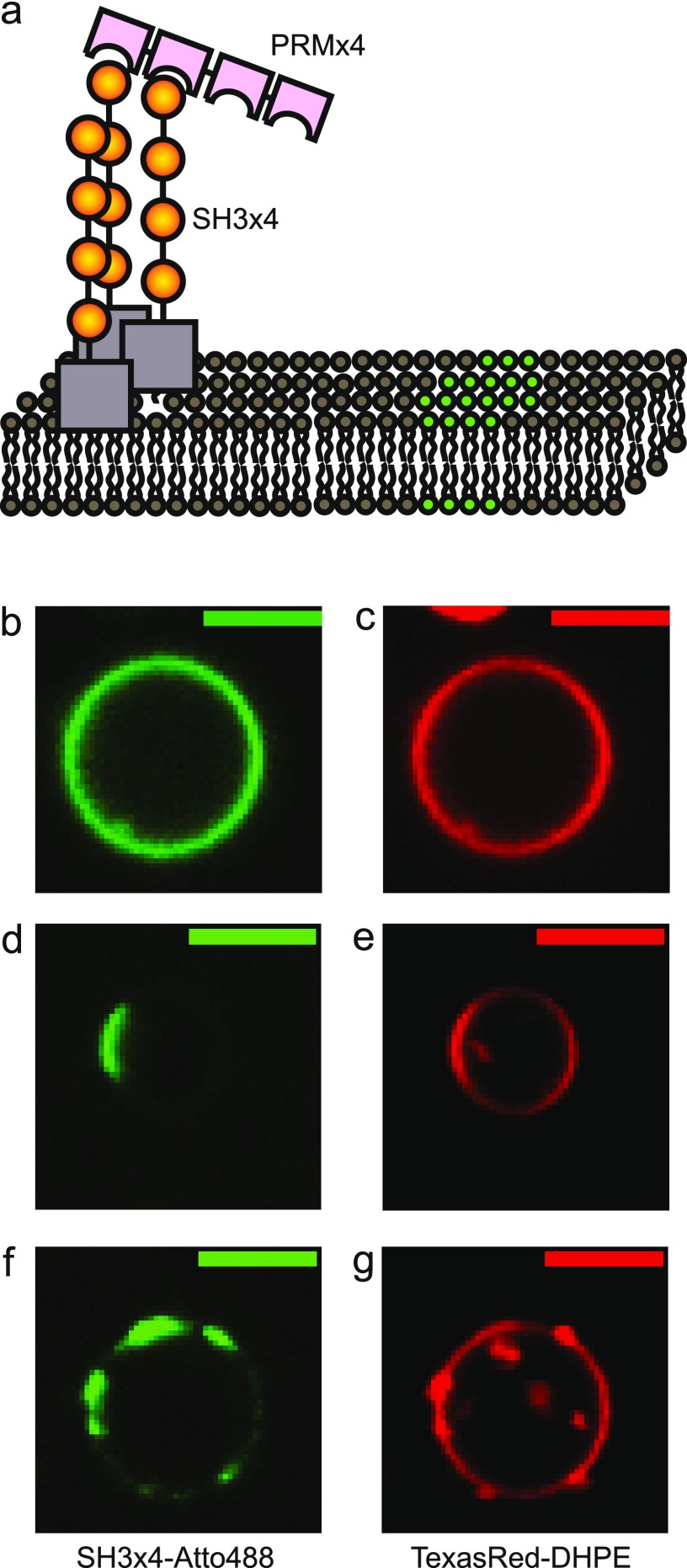
Figure 2.
Two-component phase-separating proteins on the phase-separated lipid membrane. (a) SH3 × 4 and PRM × 4 were added from solution to interact with ternary mixture GUVs with phase separation properties (green and gray for separated domains). SH3 × 4 (yellow) had a small soluble maltose binding protein (MBP, gray) domain with a polyhistidine tag, so it spontaneously anchored itself to the membranes by strong binding to Ni-NTA lipid. (b, c) Matching typical images of the Atto488 protein channel and the TexasRed-DHPE lipid channel of a two-phase-separated GUV. (d, e) Matching typical images of a two-phase-separated GUV. (f, g) Matching typical images of a GUV with multiple-phase domains. Scale bars are 5 μm. Lipid compositions were 45% DOPC, 20% DPPC, 35% cholesterol, 5% DOPS, 10% Ni-DGS, 0.1% TR-DHPE.
We incubated the ternary mixture of GUVs with different concentration combinations of SH3 × 4 and PRM × 4. GUVs were observed after 30 min of incubation. After incubation, the GUVs were statistically analyzed by taking multiple z-stacks of confocal images by both the protein and the lipid fluorescence channels. Typical examples of confocal section images are shown in Figure 2b–g. Uniform GUVs showed the same overlapping fluorescence in both channels. Phase-separated GUVs showed segregated fluorescence, where protein and lipid fluorescence usually overlapped well. Both TR-DHPE and Ni-NTA protein anchoring lipids preferred the ld domain of binary lipid phase separation.34 We mainly used protein fluorescence images to categorize the states of different GUV phases, whereas lipid channel images were used to confirm the observation and also to ensure that the GUVs were suitable for the analysis. Vesicles with multiple-phase domains, or a number of distinct domains, were categorized as microdomain–small multiple domains–vesicles because such a state is known to be stable when the line tension between two domains is relatively low.35,36
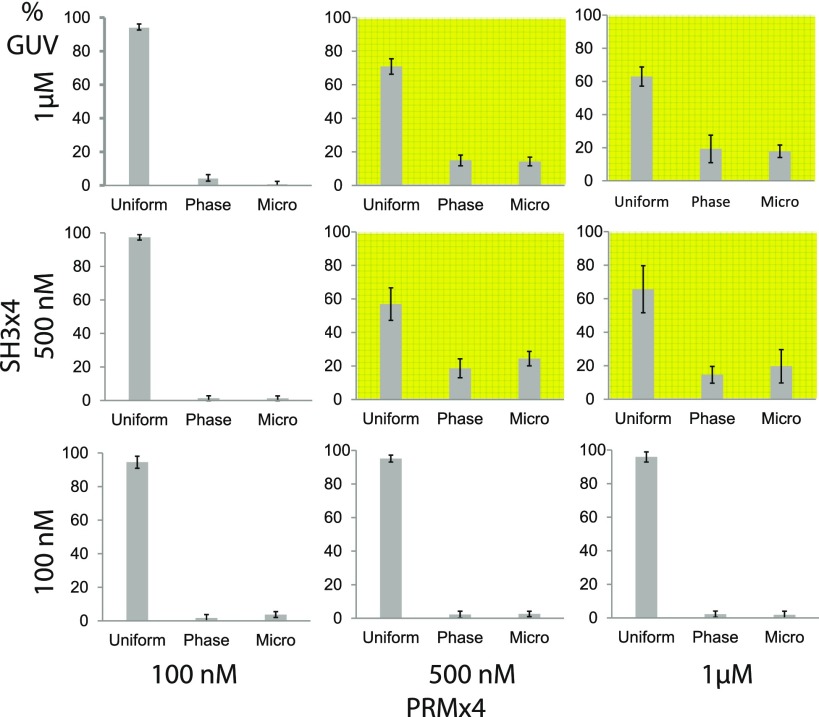
Figure 3 shows that the ratio of uniform GUVs above the threshold concentration of 500 nM showed a significant decrease compared to the GUVs below the threshold concentration due to the strong interprotein interaction of SH3 × 4 and PRM × 4 on the membrane. Shaded subfigures are for conditions where concentrations for both SH3 × 4 and PRM × 4 were the same or above 500 nM, where we saw a higher ratio of phase-separated vesicles. Nonshaded subfigures are for conditions where at least one protein concentration was below the threshold concentration, 500 nM. In the condition below the threshold concentration, most vesicles were uniform without phase separation. This means that the two-component proteins with inherent phase separation properties modulated the phase behavior of the underlying lipid bilayer by increasing the overall phase separation at high enough concentrations. Note that only SH3 × 4 was anchored to the membrane while PRM × 4 interacted by binding to SH3, and it rules out the possibility that it was the high concentration of polyhistidine-tagged proteins anchored to the membrane that caused the change in phase behavior. This was also the mode of interaction in previously reported protein-driven phase domains on the membrane, where only one component of the phase-separating proteins was anchored to the membranes, while other components were interacting from the solution.29−31
Figure 3.
Statistical distribution of resulting phase behaviors of GUVs with different concentrations of SH3 × 4 and PRM. Final phase states of GUVs after incubation with the two-component protein system at different combinations of proteins, 3 by 3, were quantified by analyzing z-stack images of well-defined GUVs. As indicated by the shaded background, it was only when concentrations of both proteins were 500 nM or higher that the phase distribution was shifted significantly toward the direction of more phase-separated vesicles. Error bars are standard deviations of multiple z-stacks analyzed. Each image covered the area of 212 × 212 μm2 with >20 vesicles. The data are averages of six z-stacks.
This observation suggests that membrane–protein interaction on the living cell plasma membranes, assisted by the raft composition of the lipid membrane, may be able to form domains as a result of the interaction between lipid and protein phase separations. The fact that both interactions coexist on the plasma membranes indicates that membrane-assisted phase separation may occur even when the condition is not strictly favorable for lipid-only- or protein-only-driven phase separation because the overall system may still be in a state of spontaneous phase separation.
One-Component Phase-Separating Proteins Increase Phase Separation in a Manner Dependent on Lipid Composition
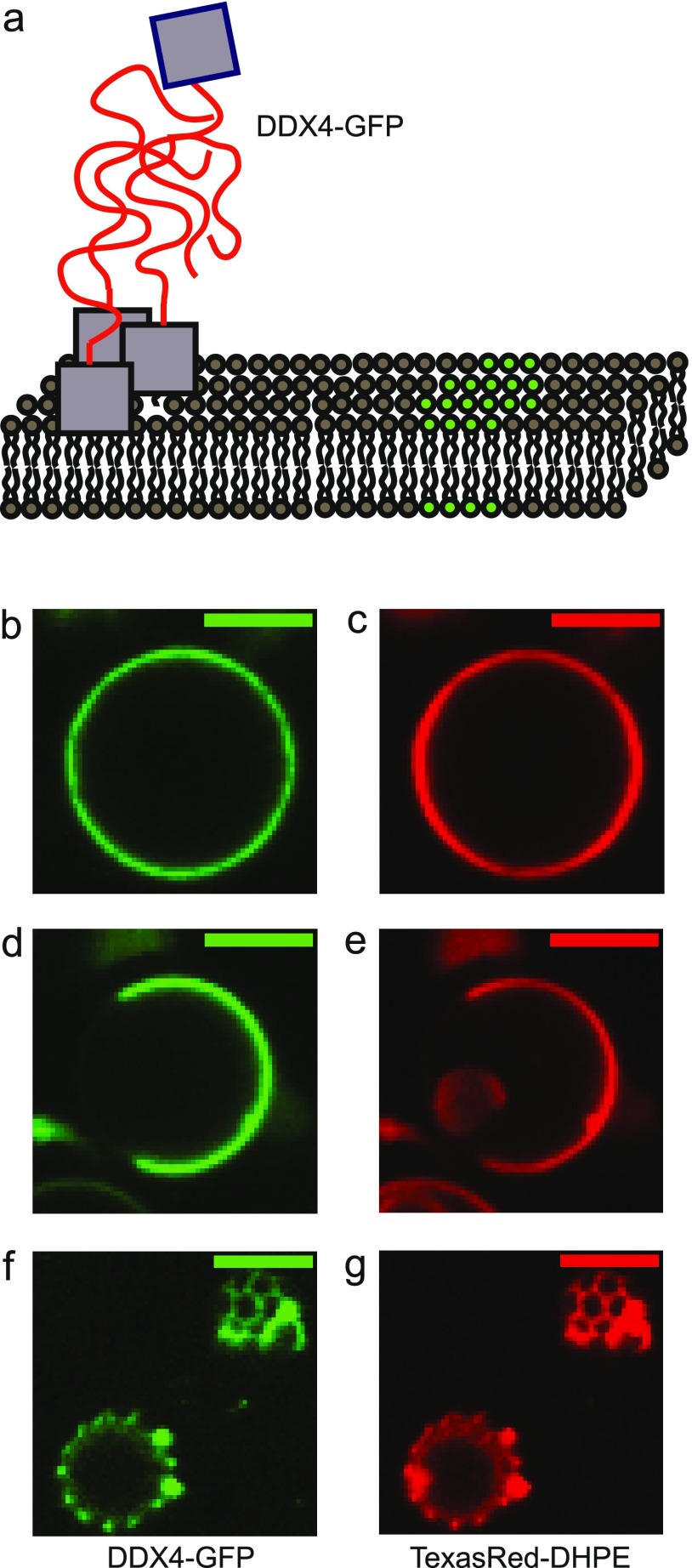
After observing the increased phase domains by the two-component system, we wanted to find out whether this was a general rule for other similar proteins with phase separation property on the membranes. We performed similar experiments with an artificially membrane-anchored IDR of DDX4 protein, which we call a one-component system in this paper (Figure 4a). The IDR of DDX4 is known to favorably interact with itself. At a high enough concentration, it forms protein-driven phase domains in solution, which are responsible for the formation of membraneless organelles in living cells37 (Supporting Information, Figure S2). To the best of our knowledge, no study has yet reported a physiological example of a one-component system on the membrane. This artificially anchored one-component system should, therefore, be considered as a model system with a minimal number of proteins used to reconstruct general phase behavior on the membranes.
Figure 4.
One-component phase-separating proteins on the phase-separated lipid membrane. (a) DDX-GFP proteins were added from solution to interact with ternary mixture GUVs with phase separation properties (green and gray for separated domains). Intrinsically disordered DDX domain (orange) was continued from the fluorescent GFP (gray) domain with a polyhistidine tag, so it spontaneously anchored itself to the membranes by strong binding to Ni-NTA lipid. (b, c) Matching typical images of the GFP protein channel and the TexasRed-DHPE lipid channel of a two-phase-separated GUV. (d, e) Matching typical images of a two-phase-separated GUV. (f, g) Matching typical images of a GUV with multiple-phase domains. Scale bars are 5 μm. Lipid compositions were 42% DOPC, 29% DPPC, 14% cholesterol, 5% DOPS, 10% Ni-DGS, 0.1% TR-DHPE for (b)–(e) and 10% DOPC, 50% DPPC, 25% cholesterol, 5% DOPS, 10% Ni-DGS, 0.1% TR-DHPE for (f) and (g).
Similar to two-component systems, one-component DDX4 at high concentration (>100 μM) could form liquid droplets in solution. Proteins at 1 μM formed a homogeneous immobile protein layer instead of fluidic protein-driven phase domains on the control glass-supported lipid bilayer as a result of strong interprotein interaction (Supporting Information, Figure S2). Ternary mixture GUVs with inherent, lipid-driven phase separation behavior were generated and observed before and after incubation with the 1 μM DDX4 proteins. The DDX4 protein had a polyhistidine tag to anchor itself to the membrane. The lipid composition was similar to the lipid mix of the two-component experiments but was a bit lower in DOPC and cholesterol,10 which enhanced the TR-DHPE fluorescence contrast between the two domains (42% DOPC, 29% DPPC, 14% cholesterol, 5% DOPS, 10% Ni-DGS, 0.1% TR-DHPE). Protein and lipid fluorescence overlapped very well after protein incubation and showed similar behavior to uniform, two-phase separation and microdomains (Figure 4b–g). Statistical distributions of the GUV state before and after protein incubation were quantified.
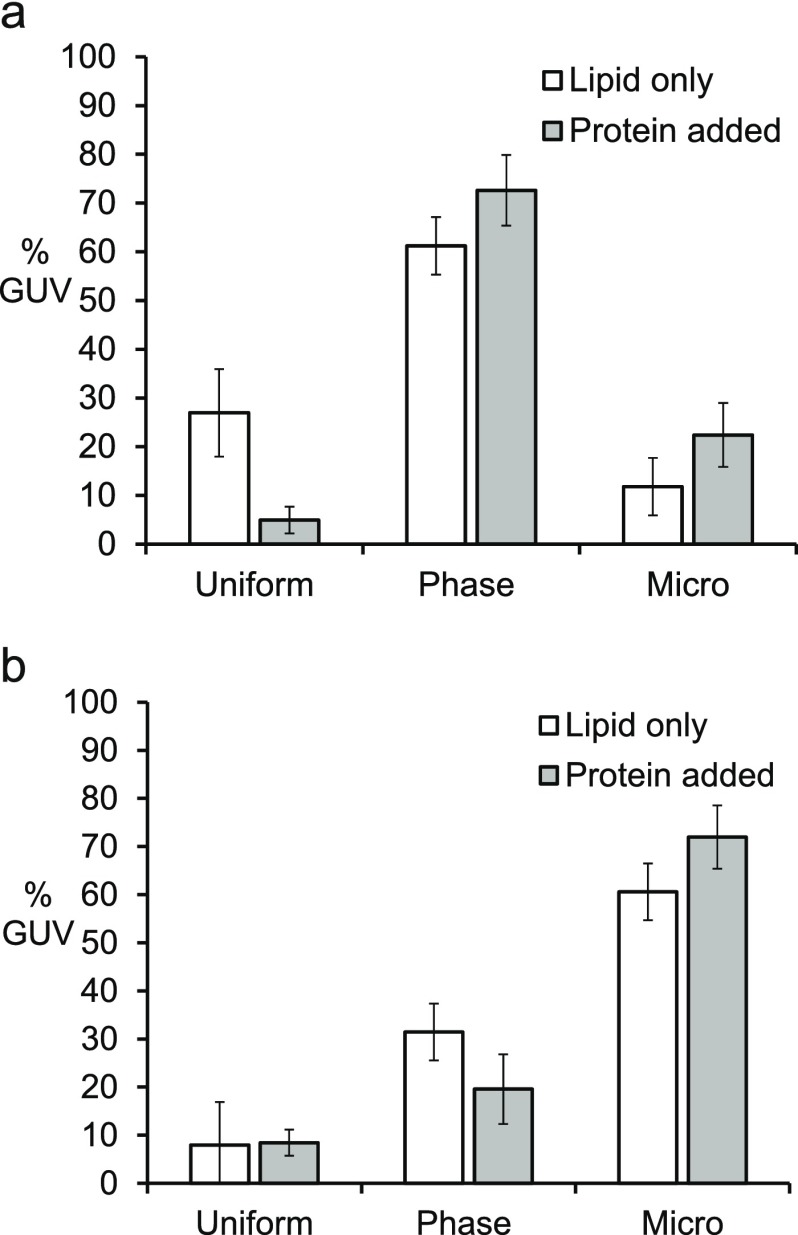
As shown in Figure 5a, the one-component interaction of the DDX protein decreased the ratio of uniform GUVs, thus increasing the ratio of phase-separated GUVs. This suggests that proteins on the membrane with strong interprotein interaction can, in general, shift lipid phase behavior toward increased phase separation. Ratios for both two-phase states and microdomains increased, which may be a natural result of the decrease in uniform vesicles. Although we did not see any clear evidence to suggest a dramatic change in the ratio between a two-phase state and a microdomain, we categorized all clear phase separation with multiple domains as microdomains to distinguish them from a simple two-phase state.
Figure 5.
Statistical distribution of GUV phase behaviors before and after interacting with one-component protein DDX. (a) Ratio of phase-separated GUV increased after one-component DDX protein incubation. The lipid compositions were 45% DOPC, 20% DPPC, 35% cholesterol, 5% DOPS, 10% Ni-DGS, 0.1% TR-DHPE. (b) For a different lipid composition, the ratio of GUV phase states did not change significantly after the protein incubation. The lipid compositions were 10% DOPC, 50% DPPC, 25% cholesterol, 5% DOPS, 10% Ni-DGS, 0.1% TR-DHPE for (f) and (g).
We also tested another lipid composition with less DOPC (10% DOPC, 50% DPPC, 25% cholesterol, 5% DOPS, 10% Ni-DGS, 0.1% TR-DHPE). This composition was also along the tie line of the ternary mixture phase diagram from a previous study.38 When a composition is along the tie line, the composition of each separated domain is supposed to be the same but their relative areas are different. A relatively less ordered, or ld, domain is supposed to be smaller in this composition. We note that it may not lie strictly on the exact tie line because our composition was not identical to the one reported previously, which did not include charged lipid and functionalizing lipid. However, we expected them to be close to the tie line as the charged lipid DOPS and Ni-NTA that we introduced had the same unsaturated carbon tail structure as the lipid DOPC. When we repeated the protein interaction experiment with this GUV composition, we could not see clear differences before and after adding the one-component DDX4 proteins (Figure 5b). This suggests that a protein-driven increase of phase separation may not be universally applicable to all lipid compositions and may be dependent on the composition, to some extent. This could be due to the fact that the same interprotein interaction may or may not cause significant disruption to make a transition in lipid phase behavior in a different lipid mixture. It is also worth mentioning that the GUVs in the composition tested later were mostly phase-separated already before interacting with proteins, leaving only limited room for an increase in phase separation.
Two-Dimensional (2D) Ising Model Simulations Show That Coupled 2D Layers with Phase Separation Property Will Increase the Overall Phase Separation of the System
We used a 2D Ising model system to help explain our observation of increased phase separation by the proteins with inherent phase separation property. As lipid membranes and proteins are both quite large molecular systems to simulate ab initio, many different models have been introduced with an emphasis on specific physical properties of interest. These include all-atomic models,11,39 coarse-grained models,40 mechanical models,41 sphere Monte-Carlo models,36 and Ising models.11,42 The Ising model treats two phases as up- and down-spins. Neighboring spins with the same sign stabilize the energy of the system. Although the model omits molecular details of phase behavior, it is a great system to describe phase organization of the membranes when we are interested in properties of binary phase domains in general. In this study, we are interested in how two-phase domains, lipid domains and anchored protein domains, affect each other, and the interaction can be interpreted as a general interaction between two domains, and so molecular detail is of secondary interest. We assumed that the two layers of 2D Ising models were stacked up. The two layers were originally independent systems, but there were a discrete number, L, of linker spins that connected the two layers. When a linker spin moved in one layer, its matching linker spin had to move in the other layer as well. The linker spins represent proteins linked to the membranes via membrane anchors. The upper layer represents protein-driven phase behavior, whereas the lower layer represents lipid-driven phase behavior. To conserve the number of up- and down-spins, we used Kawasaki dynamics where spins can only move by thermal fluctuation with no spin flipping.11,42 The protein layer was set to have Jupper = 0.9 of the interparticle interaction instead of Jlower = 1.0 for the lipid layer, so it was above the phase transition temperature of the simulation. J indicates the amount of interparticle interaction energy in the Ising model and is often used without the unit for convenience. Temperature T is also used without unit by defining it relative to a reference value. See Method and Supporting Information for a detailed simulation procedure.
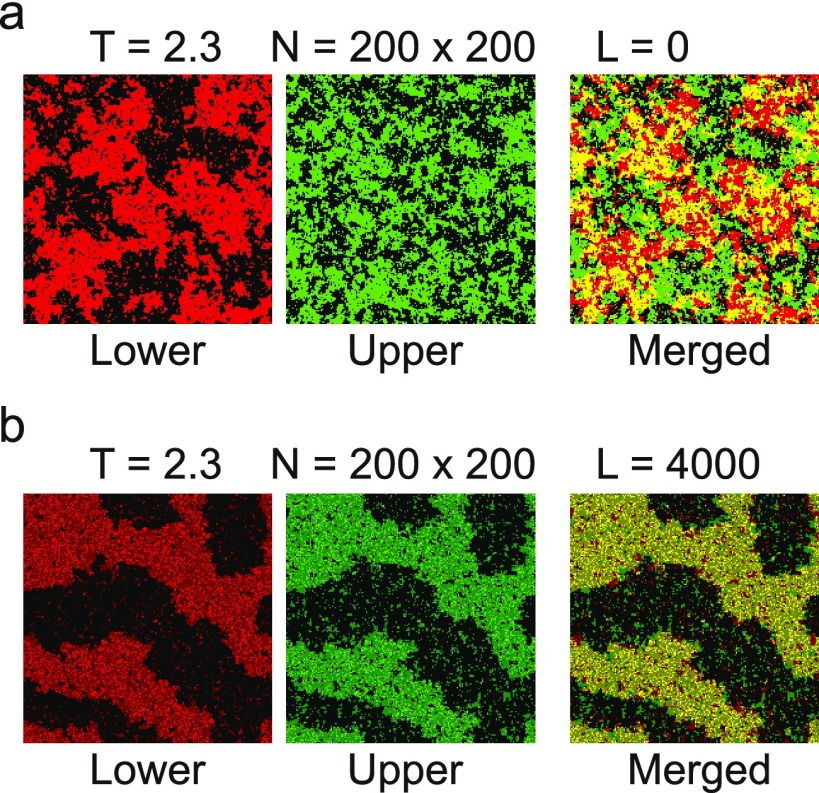
At the simulated temperature, T = 2.3, which was the expected original critical temperature of the lower layer and was also above the original transition temperature of the upper layer, we saw, as expected, independent fluctuation of both layers when the two layers were completely independent (Figure 6a). At its critical point, the lower layer showed typical critical behavior of correlated fluctuation.43 The upper layer was above its critical temperature due to lower interparticle stabilization and showed more homogeneous fluctuation. When 10% of the spins were linked using linker spins in the same condition, the two systems showed a strongly correlated fluctuation, as evidenced by the larger area of phase overlap between the two layers (Figure 6b). The entire system of two layers also became more phase-separated with less homogeneous mixing of the two spins. This indicates that coupling the two layers with independent phase behaviors via linker species lowered the transition temperature of the whole system, promoting greater phase segregation in both layers. Therefore, our observations of one-component and two-component protein systems interacting with phase-separated membranes can be understood as an example of a case of coupled layers with phase-separating properties. More simulation data with different parameters can be found in the Supporting Information (Figure S3).
Figure 6.
Layered two-dimensional (2D) Ising model simulations show increased phase separation when two layers are linked by linkers. Spin states of a simulation after t = 2 000 000 steps. For the lower layer, black and red colors indicate two spins. For the upper layer, black and green colors indicate two spins. T = 2.3, which was the critical temperature for the lower layer, and the upper layer was above its critical temperature due to lower interparticle stabilization energy (0.9 of the lower layer). N, or the total number of spins, were 40 000 for each layer. (a) Spin states for when L, the number of linker spins, was zero. Two layers showed independent fluctuations without much segregation behavior. (b) Spin states when L, the number of linker spins, was 4000, which was 10% of the total number of spins. Both layers are clearly segregated into distinct phases and two layers are moving in a correlated manner. It shows that the link between two layers can promote more phase segregation of the overall system.
Three-Component Phase-Separating Proteins with Preformed Phase Domains Can Generate More Complex Molecular Segregation
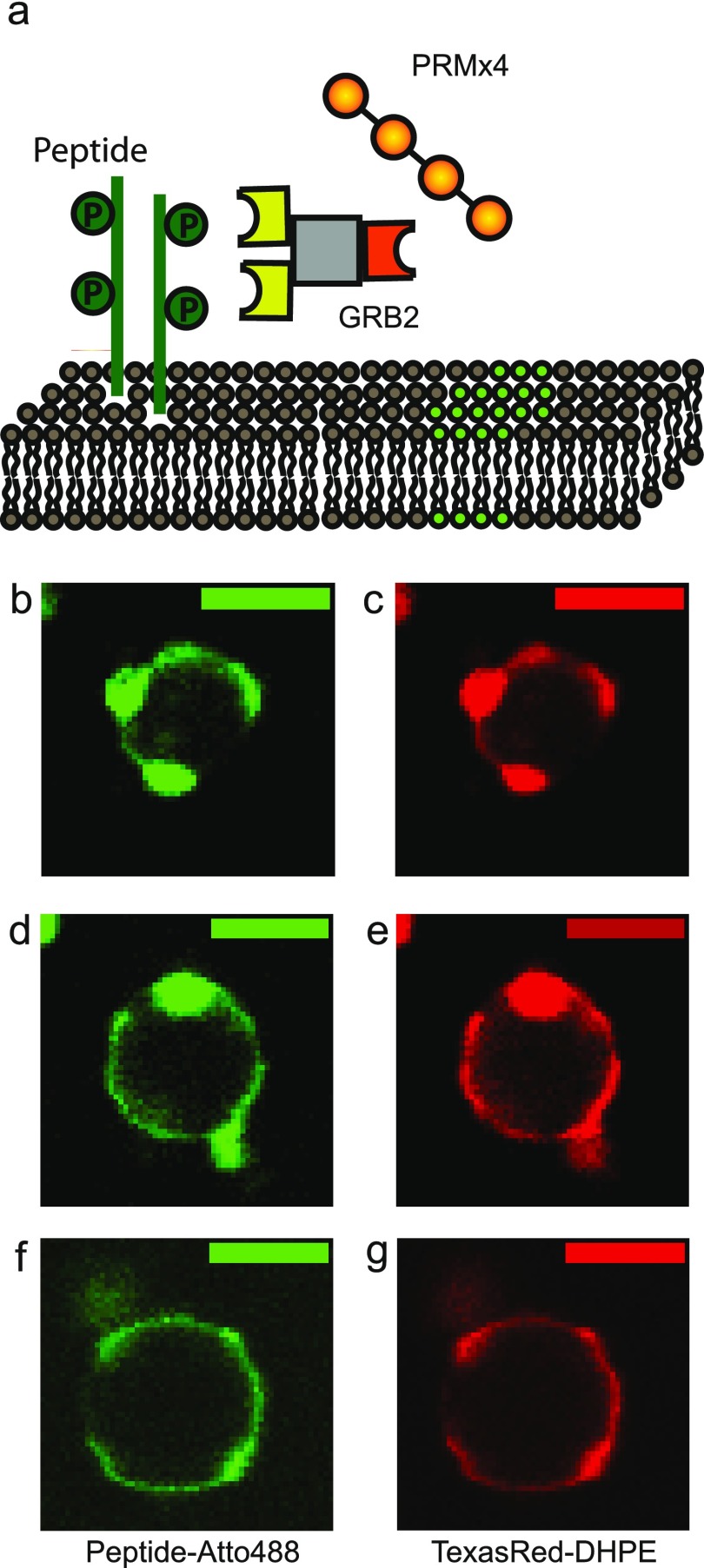
We further increased the number of proteins involved to three to study their interaction with the phase-separated lipid membranes. The three-component system is so far the only system with reported 2D phase separation behavior on the membrane.29−31 We used the protein system involved in T-cell signaling, but the LAT protein was replaced with a simple peptide, which had a sequence of a part of LAT with two phosphorylation sites (Figure 7a). On a supported lipid bilayer, the system was not able to form dynamic protein domains on the membrane, as reported previously, possibly due to the fact that our peptide mimics only a portion of the original LAT protein. However, it formed a relatively static network of domains that were in equilibrium with a pool of proteins. The system also formed protein-driven phase domains, or liquid droplets, readily in solution (Supporting Information, Figure S4).
Figure 7.
Three-component phase-separating proteins on the phase-separated lipid membrane. (a) PRM × 4, GRB2-GST, and LAT sequence peptide were added from solution to interact with ternary mixture GUVs with phase separation properties (green and gray for separated domains). LAT sequence peptide (green) was labeled with Atto488 at the C-terminal and had a polyhistidine tag at N-terminal, so it spontaneously anchored itself to the membranes by strongly binding to Ni-NTA lipid. (b–g) Matching typical images of the Atto488 protein channel and the TexasRed-DHPE lipid channel of GUVs with multiple-phase domains. Scale bars are 5 μm. Lipid compositions were 42% DOPC, 29% DPPC, 14% cholesterol, 5% DOPS, 10% Ni-DGS, 0.1% TR-DHPE.
We performed a similar experiment by mixing 5 μM of each protein into the phase-separating GUVs with the same composition, as used for the first experiment with the one-component system (42% DOPC, 29% DPPC, 14% cholesterol, 5% DOPS, 10% Ni-DGS, 0.1% TR-DHPE). The three-component system had a strong tendency to form protein-driven phase droplets. As a result, in the experimental conditions, we were able to see the protein mixture solution already becoming murky, which is a sign of protein droplet formation, immediately after mixing the three components into ∼25 μM. This solution was diluted five times into the GUV solution to allow for interaction. This meant that we observed the effect of preformed protein domains interacting with the lipid membranes with phase separation in addition to the effect of proteins with inherent phase separation properties. Even similar fluidic protein domain droplets might have different properties in terms of kinetic and thermodynamic properties,44 and we note that the protein droplets were stable for up to 2 h during our observation.
After the incubation, we observed typical GUVs with multiple-phase domains, as shown in Figure 7b–g. Most vesicles had multiple domains with uneven fluorescence intensity, giving a ratio of phase-separated vesicles above 90%, an increase from the original 70% (Figure 5a, before the protein incubation). We were not able to distinguish a pure 2D interaction on the membrane or interaction with 3D protein domains. Due to this ambiguity, we did not perform the same categorization of vesicle states that we did for other protein systems. The three-component system, interestingly enough, showed what looked like a higher-order organization by 3D protein domains. We could see the existence of binary phases around the contour of the lipid membranes and, in addition, we were often able to see the existence of a much brighter region related to interaction with the 3D protein domains. This suggests that when conditions are right, 3D protein domains interacting with the lipid membrane may be able to provide an additional molecular sorting platform. The cell cytosol is extremely crowded, and membrane–protein interaction is never limited to the 2D interaction in living cells. Accordingly, this is a possible mechanism for physiological sorting processes.
Conclusions
We reconstituted interactions between lipid membranes and proteins with phase separation behavior. One- and two-component protein systems promoted phase domain formation on GUVs with ternary mixture lipid compositions with relatively high ratios of unsaturated phospholipid DOPC. A coupled, 2D Ising model simulation showed that this can be understood as a natural result of two layers with phase separation property coupled by linker molecules. A three-component protein system with preformed protein domains interacted with the ternary mixture lipid GUVs to generate GUVs with multiple domains on the membranes. 3D phase domains interacting with a 2D lipid membrane offered a potential mechanism for a more complex sorting process on the cell membranes.
Our study suggests that coexisting phase separation properties of proteins and lipids will interact with each other, changing the final phase separation outcome of the entire system, and we should consider the combined effect of both. As living cell membranes, specifically mammalian plasma membranes, are well known to have inherent phase separation properties,9,45 we should expect enhanced protein-driven phase separation on the lipid membrane bilayer. Acknowledging the enhanced phase separation on the membrane should help us to interpret and engineer molecular sorting processes in the future.
Caution should be exercised when translating our in vitro experimental data to living cell membranes, and we wanted to highlight a few important points. First, living cell membranes are far more complex than our artificial membrane system. Living cell membranes contain a lot more lipids and transmembrane proteins,46 and membrane proteins are often dynamically interacting with cytosolic proteins such as actin cytoskeleton.47 Therefore, it is important to realize that the thermodynamic property of simple artificial membrane systems may not appear exactly as expected in living cell membranes.15 Second, living cell membranes are asymmetric. Lipid bilayers are composed of two leaflets, and many living cell membranes, including the plasma membrane, actively maintain the compositions of leaflets in an asymmetric manner.48 Asymmetric distribution of glycolipids may further force the movement of cholesterol from one leaflet to the other.49 GUV bilayers are symmetric, and the effect of asymmetric lipid distribution is not included in our study, while asymmetric membranes may result in a more complicated phase behavior.50 Lastly, some major components in cell plasma membranes, such as sphingomyelin and ganglioside that are missing in our reconstitution, are known to play important roles in membrane organization. Glycolipids, for example, may itself interact with other molecules such as extracellular matrix and GPI membrane anchors by the carbohydrate groups, changing the overall phase behavior and organization.51,52
Methods
GUV Preparation
A total net mass of between 0.2 and 0.5 mg of lipid mixture of the desired composition in chloroform was mixed in a clean round-bottom flask. Chloroform was then removed by rotavap under vacuum at 50 °C for >30 min to generate a uniform lipid film. The film was further dried by clean nitrogen blown for 5 min. Then, 1 mL of sucrose solution (at a concentration of 220 mM to match osmotic balance with the buffer) was added to incubate overnight at 37 °C. After incubation, the GUV sample was centrifuged at 11 500 RPM for 15 min at room temperature to remove aggregated lipid species. Supernatant was taken and stored at 4 °C before use. All lipids were purchased from Avanti Polar Lipids.
Protein Purification
Most proteins discussed in this paper were overexpressed in E. coli BL21 DE3 (Sigma-Aldrich) to affinity purify by polyhistidine or GST tag, and appropriate further purification steps were taken as needed. Briefly, SH3 × 4 and PRM × 4 were purified by steps that were similar to the previous protocol.20 The plasmids were generous gifts from the Rosen lab (UT Southwestern). After overexpression in E. coli, proteins were affinity purified by Ni-NTA column and further purified by ion exchange column (SP Sepharose FF 5 mL and HiTrap Q HP 5 mL, GE Healthcare) and size exclusion column (Superdex 75, GE Healthcare). The PRM × 4 were cleaved enzymatically to remove unwanted MBP-polyhistidine tags. Further purification was performed to separate out tags. The DDX4-GFP gene was synthesized and introduced into a plasmid based on the original sequence (GenScript). E. coli was grown at 37 °C for 3–5 h to overexpress by IPTG (Sigma-Aldrich) 1 mM RT overnight. Harvested cells were sonicated and centrifuged to affinity purify by Ni-NTA (Thermo Fischer Scientific). Imidazole solution (300 mM) in 20 mM Hepes buffer, pH 7.4, 300 mM NaCl was used for elution. Imidazole solution (3 mM) in the same buffer was used for washing. The protein was finally purified by desalting column (HiTrap 5 mL, Invitrogen). Proteins were centrifuged before use to remove potential aggregates. The GRB2 gene was obtained from AddGene, which was deposited by Vale lab.29 GRB2 plasmid-introduced E. coli was grown at 37 °C for 3–5 h to overexpress by IPTG 0.5 mM RT overnight. Harvested cells were sonicated and centrifuged to affinity purify by GST resins (Thermo Fischer Scientific). Glutathione solution (10 mM) in 20 mM Hepes pH 7.4, 2 mM β-mercaptoethanol, and 150 mM NaCl was used for elution, and the same buffer solution without glutathione was used for washing. The protein was desalting column purified to remove excess glutathione. For the reported experiments, we used the purified GRB2 protein as it is, but we also tried experiments with the GST-tag-removed GRB2 by enzymatic digestion. Here, there was no difference in the observed behavior of protein phase formation. The peptide was synthetized by Biomatik. Its sequence is given in the Supporting Information. Two phosphate groups were introduced as part of the synthesis process, and the peptide was used within 2 h of thawing to prevent potential loss of the phosphate group.
Protein Labeling
SH3 × 4 and LAT-like peptide were fluorescently labeled by Atto488-maleimide (Invitrogen) using a cysteine residue introduced for the purpose of site-specific labeling. Protein solution was incubated with an equal concentration of Atto488-maleimide overnight at 4 °C in 20 mM Hepes, pH 7.4, 100 mM NaCl buffer, which was also the standard buffer for most of the experiments in this paper. Labeled proteins were purified by desalting to remove unbound dyes. The typical labeling ratio varied from 20 to 60%, which was measured by Nanodrop (Thermo Fischer Scientific).
GUV-Protein Sample Imaging
A home-built stainless steel holder that could enclose a round cover glass at the bottom was used for imaging. An open chamber was made of >1 mL volume above the cover glass to add any solution for imaging. The holder was cleaned by bath sonication for 30 min in 1% detergent solution and then sonicated for 30 min in isopropyl alcohol and water at a 1:1 ratio. After assembling the cover glass and the holder, the glass surface was blocked by incubating with 1 mg/mL BSA solution for 30 min. After incubation, the chamber was washed five times with buffer to add 10–50 μL of GUV solution for imaging.
To analyze protein interaction, the desired concentrations of proteins were added by pipette injection and were mixed gently. To ensure quick and homogeneous interaction, the protein solution was added at a comparable volume to the volume of solution in the chamber (20–50% by volume).
A Nikon Eclipse C1 confocal fluorescence microscope system was used for imaging. Lasers (488 and 543 nm) were used for excitation of Atto488/GFP and TexasRed, respectively. The Nikon Plan Apo VC 60X WI Water objective was used for imaging. Automatic z position control was used for precise z-stack acquisition and x, y were controlled manually. FIJI (ImageJ) was used for image analysis.
Ising Model Simulation
A Kawasaki dynamics, 2D Ising model Monte-Carlo simulation was performed following a general method.42 Matlab (MathWorks) software was used for the simulation. Spins (200 × 200) were simulated, with half the spins being up-spins and half down-spins in one layer. Two layers of simulations were run simultaneously. All spins were initialized by randomly mixing spins at a very high temperature. Each time step performed 80 000 trials of moving particles by one position. Energy change was calculated for each potential movement. When neighboring spins had the same sign, it contributed −J = −1.0 of stabilization. Upper and lower layer spins were not assumed to interact directly. If the new state was lower in energy, the move was accepted. If not, the new state was accepted by probability exp(−ΔE/T). If the selected spin was one of the linker spins, which span both layers, the energy contribution from both layers was calculated, and the linker spin moved in both layers when the movement was accepted. All linker spins were assumed to be up-spins. A total of 2 000 000 steps was performed for each simulation. The critical temperature or transition temperature of the 2D Ising model with J = 1.0 is Tc = 2.3. In our layered simulation, Jlower = 1.0 and Jupper = 0.9, which means the lower layer was at its original critical temperature at T = 2.3, and the upper layer was still above at its original critical temperature at T = 2.3. It took 1.3 days to complete one simulation by a 2.8 GHz CPU. A detailed stepwise simulation method and the definition of a unit step can be found in the Supporting Information.
Acknowledgments
The authors would like to thank the Rosen lab (UT Southwestern) for providing the plasmids, Dr. Margarete Stratton for helpful discussion of protein purification, Bob Peaslee for manufacturing the sample chambers and microscope adapters, and Amy Replogle of the Science Core Facility for microscope maintenance and training. M.Y.I. acknowledges the Gilbertson scholarship award and summer research grants in science and mathematics from the University of Puget Sound. E.H.M. acknowledges a Washington NASA space grant scholarship award and summer research grants in science and mathematics from the University of Puget Sound. The research was supported by the Department of Chemistry at the University of Puget Sound.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b00327.
Fluorescence recovery after photobleaching shows partially mobile lipid membrane after sequential incubation of SH3 × 4 and PRM × 4 (Figure S1); DDX4-GFP forming 3D droplets in solution and immobile lipid membrane on SLB (Figure S2); additional Ising model simulation (Figure S3); three-component protein system on the supported lipid bilayer (Figure S4) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Simons K.; Ikonen E. Functional Rafts in Cell Membranes. Nature 1997, 387, 569–572. 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Lingwood D.; Simons K. Lipid Rafts As a Membrane-Organizing Principle. Science 2010, 327, 46–50. 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- Ikonen E. Roles of Lipid Rafts in Membrane Transport. Curr. Opin. Cell Biol. 2001, 13, 470–477. 10.1016/S0955-0674(00)00238-6. [DOI] [PubMed] [Google Scholar]
- Groves J. T.; Kuriyan J. Molecular Mechanisms in Signal Transduction at the Membrane. Nat. Struct. Mol. Biol. 2010, 17, 659–665. 10.1038/nsmb.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillemeier B. F.; Mörtelmaier M. A.; Forstner M. B.; Huppa J. B.; Groves J. T.; Davis M. M. TCR and Lat Are Expressed on Separate Protein Islands on T Cell Membranes and Concatenate during Activation. Nat. Immunol. 2010, 11, 90 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaita K.; Nair P. M.; Petit R. S.; Neve R. M.; Das D.; Gray J. W.; Groves J. T. Restriction of Receptor Movement Alters Cellular Response: Physical Force Sensing by EphA2. Science 2010, 327, 1380. 10.1126/science.1181729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M. B.; Shelby S. A.; Núñez M. F.; Wisser K.; Veatch S. L. Protein Sorting by Lipid Phase-like Domains Supports Emergent Signaling Function in B Lymphocyte Plasma Membranes. eLife 2017, 6, e19891 10.7554/eLife.19891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart T.; Hunt G.; Farkas E. R.; Webb W. W.; Feigenson G. W. Fluorescence Probe Partitioning between Lo/Ld Phases in Lipid Membranes. Biochim. Biophys. Acta, Biomembr. 2007, 1768, 2182–2194. 10.1016/j.bbamem.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart T.; Hammond A. T.; Sengupta P.; Hess S. T.; Holowka D. A.; Baird B. A.; Webb W. W. Large-Scale Fluid/Fluid Phase Separation of Proteins and Lipids in Giant Plasma Membrane Vesicles. Proc. Natl. Acad. Sci. USA 2007, 104, 3165–3170. 10.1073/pnas.0611357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch S. L.; Keller S. L. Separation of Liquid Phases in Giant Vesicles of Ternary Mixtures of Phospholipids and Cholesterol. Biophys. J. 2003, 85, 3074–3083. 10.1016/S0006-3495(03)74726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.-H.; Saha S.; Polley A.; Huang H.; Mayor S.; Rao M.; Groves J. T. Live Cell Plasma Membranes Do Not Exhibit a Miscibility Phase Transition over a Wide Range of Temperatures. J. Phys. Chem. B 2015, 119, 4450–4459. 10.1021/jp512839q. [DOI] [PubMed] [Google Scholar]
- Rayermann S. P.; Rayermann G. E.; Cornell C. E.; Merz A. J.; Keller S. L. Hallmarks of Reversible Separation of Living, Unperturbed Cell Membranes into Two Liquid Phases. Biophys. J. 2017, 113, 2425–2432. 10.1016/j.bpj.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier N. C.; Masters T. A.; Sheetz M. P. Mechanical Feedback between Membrane Tension and Dynamics. Trends Cell Biol. 2012, 22, 527–535. 10.1016/j.tcb.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Portet T.; Gordon S. E.; Keller S. L. Increasing Membrane Tension Decreases Miscibility Temperatures; an Experimental Demonstration via Micropipette Aspiration. Biophys. J. 2012, 103, L35–L37. 10.1016/j.bpj.2012.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z.; Graber Z. T.; Baumgart T.; Stone H. A.; Cohen A. E. Cell Membranes Resist Flow. Cell 2018, 175, 1769–1779.e13. 10.1016/j.cell.2018.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike L. J. Rafts Defined: A Report on the Keystone Symposium on Lipid Rafts and Cell Function. J. Lipid Res. 2006, 47, 1597–1598. 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- Hyman A. A.; Weber C. A.; Jülicher F. Liquid-Liquid Phase Separation in Biology. Annu. Rev. Cell Dev. Biol. 2014, 30, 39–58. 10.1146/annurev-cellbio-100913-013325. [DOI] [PubMed] [Google Scholar]
- Shin Y.; Brangwynne C. P. Liquid Phase Condensation in Cell Physiology and Disease. Science 2017, 357, eaaf4382 10.1126/science.aaf4382. [DOI] [PubMed] [Google Scholar]
- Boeynaems S.; Alberti S.; Fawzi N. L.; Mittag T.; Polymenidou M.; Rousseau F.; Schymkowitz J.; Shorter J.; Wolozin B.; Van Den Bosch L.; et al. Protein Phase Separation: A New Phase in Cell Biology. Trends Cell Biol. 2018, 28, 420–435. 10.1016/j.tcb.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P.; Banjade S.; Cheng H.-C.; Kim S.; Chen B.; Guo L.; Llaguno M.; Hollingsworth J. V.; King D. S.; Banani S. F.; et al. Phase Transitions in the Assembly of Multivalent Signalling Proteins. Nature 2012, 483, 336–340. 10.1038/nature10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliex A.; Temirov J.; Lee J.; Coughlin M.; Kanagaraj A. P.; Kim H. J.; Mittag T.; Taylor J. P. Phase Separation by Low Complexity Domains Promotes Stress Granule Assembly and Drives Pathological Fibrillization. Cell 2015, 163, 123–133. 10.1016/j.cell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaum-Garfinkle S.; Kim Y.; Szczepaniak K.; Chen C. C.-H.; Eckmann C. R.; Myong S.; Brangwynne C. P. The Disordered P Granule Protein LAF-1 Drives Phase Separation into Droplets with Tunable Viscosity and Dynamics. Proc. Natl. Acad. Sci. USA 2015, 112, 7189. 10.1073/pnas.1504822112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.; Currie S. L.; Rosen M. K. Intrinsically Disordered Sequences Enable Modulation of Protein Phase Separation through Distributed Tyrosine Motifs. J. Biol. Chem. 2017, 292, 19110–19120. 10.1074/jbc.M117.800466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banani S. F.; Rice A. M.; Peeples W. B.; Lin Y.; Jain S.; Parker R.; Rosen M. K. Compositional Control of Phase-Separated Cellular Bodies. Cell 2016, 166, 651–663. 10.1016/j.cell.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster B. S.; Reed E. H.; Parthasarathy R.; Jahnke C. N.; Caldwell R. M.; Bermudez J. G.; Ramage H.; Good M. C.; Hammer D. A. Controllable Protein Phase Separation and Modular Recruitment to Form Responsive Membraneless Organelles. Nat. Commun. 2018, 9, 2985 10.1038/s41467-018-05403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic D.; Wu Y.; Bian X.; Camilli P. D. A Liquid Phase of Synapsin and Lipid Vesicles. Science 2018, 361, 604–607. 10.1126/science.aat5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky V. N. Intrinsically Disordered Proteins in Overcrowded Milieu: Membrane-Less Organelles, Phase Separation, and Intrinsic Disorder. Curr. Opin. Struct. Biol. 2017, 44, 18–30. 10.1016/j.sbi.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Zhu L.; Brangwynne C. P. Nuclear Bodies: The Emerging Biophysics of Nucleoplasmic Phases. Curr. Opin. Cell Biol. 2015, 34, 23–30. 10.1016/j.ceb.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X.; Ditlev J. A.; Hui E.; Xing W.; Banjade S.; Okrut J.; King D. S.; Taunton J.; Rosen M. K.; Vale R. D. Phase Separation of Signaling Molecules Promotes T Cell Receptor Signal Transduction. Science 2016, 352, 595–599. 10.1126/science.aad9964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banjade S.; Rosen M. K. Phase Transitions of Multivalent Proteins Can Promote Clustering of Membrane Receptors. eLife 2014, 3, e04123 10.7554/eLife.04123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. Y. C.; Yan Q.; Lin W.-C.; Chung J. K.; Hansen S. D.; Christensen S. M.; Tu H.-L.; Kuriyan J.; Groves J. T. Phosphotyrosine-Mediated LAT Assembly on Membranes Drives Kinetic Bifurcation in Recruitment Dynamics of the Ras Activator SOS. Proc. Natl. Acad. Sci. USA 2016, 113, 8218–8223. 10.1073/pnas.1602602113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheve C. S.; Gonzales P. A.; Momin N.; Stachowiak J. C. Steric Pressure between Membrane-Bound Proteins Opposes Lipid Phase Separation. J. Am. Chem. Soc. 2013, 135, 1185–1188. 10.1021/ja3099867. [DOI] [PubMed] [Google Scholar]
- Bigay J.; Antonny B. Curvature, Lipid Packing, and Electrostatics of Membrane Organelles: Defining Cellular Territories in Determining Specificity. Dev. Cell 2012, 23, 886–895. 10.1016/j.devcel.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Stachowiak J. C.; Schmid E. M.; Ryan C. J.; Ann H. S.; Sasaki D. Y.; Sherman M. B.; Geissler P. L.; Fletcher D. A.; Hayden C. C. Membrane Bending by Protein–Protein Crowding. Nat. Cell Biol. 2012, 14, 944. 10.1038/ncb2561. [DOI] [PubMed] [Google Scholar]
- Konyakhina T. M.; Goh S. L.; Amazon J.; Heberle F. A.; Wu J.; Feigenson G. W. Control of a Nanoscopic-to-Macroscopic Transition: Modulated Phases in Four-Component DSPC/DOPC/POPC/Chol Giant Unilamellar Vesicles. Biophys. J. 2011, 101, L8–L10. 10.1016/j.bpj.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amazon J. J.; Goh S. L.; Feigenson G. W. Competition between Line Tension and Curvature Stabilizes Modulated Phase Patterns on the Surface of Giant Unilamellar Vesicles: A Simulation Study. Phys. Rev. E 2013, 87, 022708 10.1103/PhysRevE.87.022708. [DOI] [PubMed] [Google Scholar]
- Nott T. J.; Petsalaki E.; Farber P.; Jervis D.; Fussner E.; Plochowietz A.; Craggs T. D.; Bazett-Jones D. P.; Pawson T.; Forman-Kay J. D.; et al. Phase Transition of a Disordered Nuage Protein Generates Environmentally Responsive Membraneless Organelles. Mol. Cell 2015, 57, 936–947. 10.1016/j.molcel.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch S. L.; Soubias O.; Keller S. L.; Gawrisch K. Critical Fluctuations in Domain-Forming Lipid Mixtures. Proc. Natl. Acad. Sci. USA 2007, 104, 17650. 10.1073/pnas.0703513104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit S. A.; Jakobsson E.; Scott H. L. Simulation of the Early Stages of Nano-Domain Formation in Mixed Bilayers of Sphingomyelin, Cholesterol, and Dioleylphosphatidylcholine. Biophys. J. 2004, 87, 3312–3322. 10.1529/biophysj.104.046078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risselada H. J.; Marrink S. J. The Molecular Face of Lipid Rafts in Model Membranes. Proc. Natl. Acad. Sci. USA 2008, 105, 17367. 10.1073/pnas.0807527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart T.; Das S.; Webb W. W.; Jenkins J. T. Membrane Elasticity in Giant Vesicles with Fluid Phase Coexistence. Biophys. J. 2005, 89, 1067–1080. 10.1529/biophysj.104.049692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machta B. B.; Papanikolaou S.; Sethna J. P.; Veatch S. L. Minimal Model of Plasma Membrane Heterogeneity Requires Coupling Cortical Actin to Criticality. Biophys. J. 2011, 100, 1668–1677. 10.1016/j.bpj.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengers J. V.; Sengers J. M. H. L. Thermodynamic Behavior of Fluids Near the Critical Point. Annu. Rev. Phys. Chem. 1986, 37, 189–222. 10.1146/annurev.pc.37.100186.001201. [DOI] [Google Scholar]
- Alberti S.; Gladfelter A.; Mittag T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 2019, 176, 419–434. 10.1016/j.cell.2018.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D.; Ries J.; Schwille P.; Simons K. Plasma Membranes Are Poised for Activation of Raft Phase Coalescence at Physiological Temperature. Proc. Natl. Acad. Sci. USA 2008, 105, 10005. 10.1073/pnas.0804374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K.; Gerl M. J. Revitalizing Membrane Rafts: New Tools and Insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 688. 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- Saha S.; Lee I.-H.; Polley A.; Groves J. T.; Rao M.; Mayor S. Diffusion of GPI-Anchored Proteins Is Influenced by the Activity of Dynamic Cortical Actin. MBoC 2015, 26, 4033–4045. 10.1091/mbc.E15-06-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins H. M.; Baldridge R. D.; Xu P.; Graham T. R. Role of Flippases, Scramblases and Transfer Proteins in Phosphatidylserine Subcellular Distribution. Traffic 2015, 16, 35–47. 10.1111/tra.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondelli V.; Fragneto G.; Motta S.; Del Favero E.; Brocca P.; Sonnino S.; Cantù L. Ganglioside GM1 Forces the Redistribution of Cholesterol in a Biomimetic Membrane. Biochim. Biophys. Acta, Biomembr. 2012, 1818, 2860–2867. 10.1016/j.bbamem.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Kiessling V.; Crane J. M.; Tamm L. K. Transbilayer Effects of Raft-Like Lipid Domains in Asymmetric Planar Bilayers Measured by Single Molecule Tracking. Biophys. J. 2006, 91, 3313–3326. 10.1529/biophysj.106.091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam A. B.; Guidotti G.; Manoharan V. N.; Stone H. A. Glycans Pattern the Phase Behaviour of Lipid Membranes. Nat. Mater. 2013, 12, 128. 10.1038/nmat3492. [DOI] [PubMed] [Google Scholar]
- Groves J. T. Glycans’ Imprints. Nat. Mater. 2013, 12, 96. 10.1038/nmat3555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.