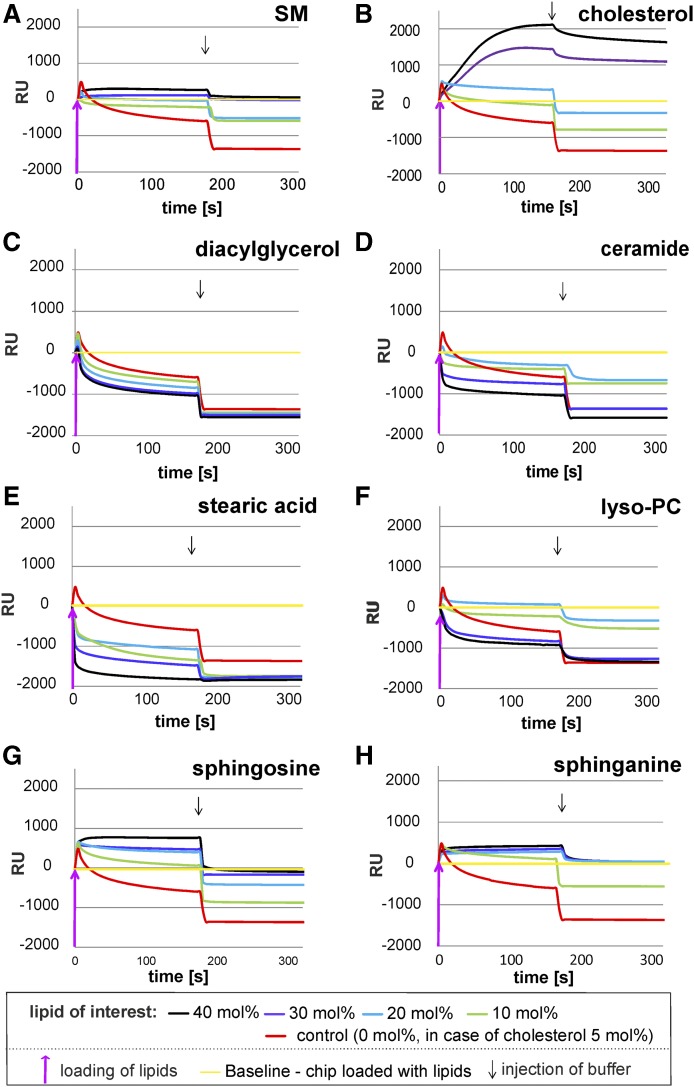
Fig. 4.
Mobilization of membrane lipids from BMP-containing, negatively charged lipid layers by GM2AP is strongly modified by the liposomal lipid composition. The binding and mobilization of membrane lipids by g-rGM2AP were investigated in SPR studies. After the immobilization of negatively charged liposomes containing 0–40 mol% lipid of interest, 20 mol% BMP, 5 mol% Chol, 10 mol% GM2, and DOPC as a host lipid, g-rGM2AP was injected into the flow cell (2.5 µM in 20 mM sodium citrate, pH 4.2) at a flow rate of 20 µl/min for 180 s. This was followed by an injection of protein-free buffer (220 s, 20 µl/min), indicated by an arrow. Curves falling below the baseline (yellow) suggest solubilization and mobilization of membrane lipids. Loading of lipids resulted in a shift of approximately 2,000 RUs, which corresponds to a lipid monolayer and was set as the baseline (RU = 0). The baseline corresponds to 100% of loaded lipid material. The RUs above the baseline (yellow) represent material bound to the lipid layer during the experiment, whereas RUs below the baseline represent material loss, which not only includes protein (GM2AP) added during the experiment but also lipid material mobilized and released from the lipid layer. Only if the RU value drops below the baseline can we be certain that lipids from the lipid layer have been removed, in amounts equal to the RU values given. The mobilization of lipids from lipid layers consisting of 0 mol% lipid of interest, 20 mol% BMP, and 5 mol% Chol by g-rGM2AP was set as the control for all experiments (red line). All lines shown are one representative out of three. BMP, DAG, Cer, stearic acid, and lyso-PC stimulated lipid mobilization by GM2AP, while SM, Chol, So, and Sa inhibited it.