Figure 3.
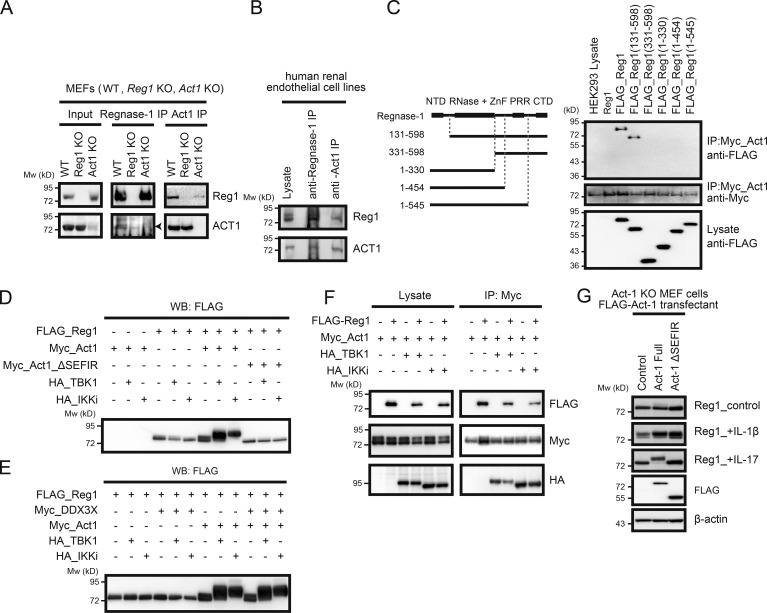
Act1 plays a pivotal role in Regnase-1 phosphorylation induced by IL-17. (A and B) Coimmunoprecipitation of Regnase-1 (Reg1) and Act1 from primary MEFs. Cell lysates from WT, Regnase-1–deficient, and Act1–deficient MEFs (A) or a human renal glomerular endothelial cell line (B) were coimmunoprecipitated by either anti–Regnase-1 or anti-Act1 antibody. Eluted proteins were analyzed with anti–Regnase-1 and anti-Act1 antibodies. (C) Coimmunoprecipitates of Act1 with full-length or N- or C-terminally truncated forms of Regnase-1. All constructs were independently expressed using HEK293 cells. A control (untagged Regnase-1) and FLAG-tagged Regnase-1 variants (left) were coimmunoprecipitated with Myc-tagged Act1. Eluted proteins were subjected to immunoblotting analysis (right) with anti-FLAG and anti-Myc antibodies. (D and E) Immunoblotting analysis of FLAG-tagged Regnase-1 in HEK293 cells transfected with FLAG–Regnase-1, Myc-tagged Act1 (D), Myc-tagged Act1 mutant (Myc-Act1 ΔSEFIR; D), Myc-tagged DDX3X (E), HA-tagged TBK1 and HA-tagged IKKi (D and E). (F) Immunoblotting analysis of Act1-deficient MEFs stably expressing FLAG-tagged Act1 (full-length and ΔSEFIR mutant). Cells were stimulated with IL-1β and IL-17 for 1 h, lysed, and analyzed by immunoblotting of Regnase-1, FLAG-Act1, and β-actin. (G) Coimmunoprecipitation of FLAG-tagged Regnase-1, Myc-tagged Act1, HA-tagged TBK1, and HA-tagged IKKi. Cell lysates from HEK293 transfectants were mixed and coimmunoprecipitated by anti–Myc-coated beads. Eluted proteins were subjected to immunoblotting analysis with anti-FLAG, anti-Myc, and anti-HA antibodies. Mw, molecular weight.