Figure 4.
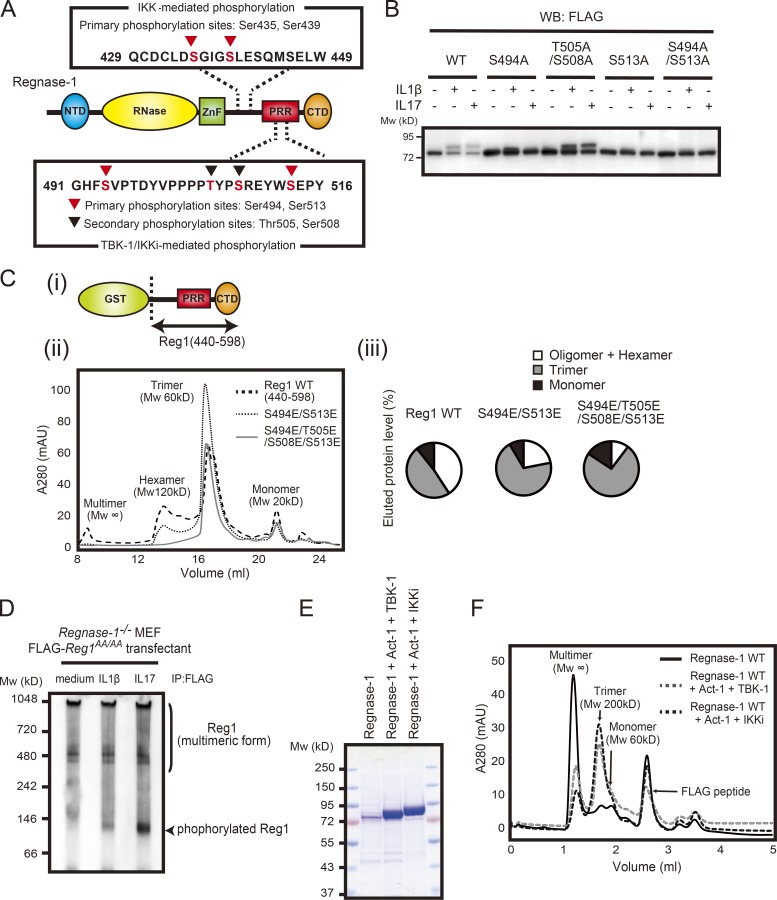
Phosphorylation of Regnase-1 results in dissociation of oligomerized Regnase-1. (A) Domain schematic of Regnase-1 and mapping of the phosphorylation sites by IKKs (IKKα and IKKβ) and TBK1/IKKi. NTD, N-terminal domain; ZnF, zinc-finger domain; PRR, proline-rich region; CTD, C-terminal domain. (B) Immunoblotting analysis of Regnase-1 in HeLa cells transfected with Regnase-1 mutants (WT, S494A, T505A/S508A, S513A, and S494A/S513A). Cells were stimulated with IL-1β (10 ng/ml) and IL-17A (50 ng/ml) for 1 h. WB, Western blotting. (C) i: Diagram of the GST-fused Regnase-1 (Reg1) construct (440–598). ii: Gel filtration of WT and mutated (S494E/S513E or S494E/T505E/S508E/S513E) Regnase-1 (440–598). The molecular weight of each elution peak was estimated by using molecular weight standard markers and defined as multimer (Mw: ∞), hexamer (Mw: 120 kD), trimer (Mw: 60 kD), or monomer (Mw: 20 kD). iii: The eluted fractions (multimer + hexamer, trimer, and monomer) were quantified as a percentage of total eluted proteins. mAU, milli-absorbance unit. (D) Native-PAGE and immunoblotting analysis of Regnase-1. Regnase-1 was obtained from Regnase-1–deficient MEFs expressing FLAG-tagged Regnase-1 AA mutant stimulated with IL-1β (10 ng/ml) and IL-17A (50 mg/ml) for 1 h. (E) SDS-PAGE of purified Regnase-1 proteins from Expi-293F (Thermo Fisher Scientific) cells transiently expressing FLAG-tagged Regnase-1 with Myc-tagged Act1 and HA-tagged TBK1 or HA-tagged IKKi. Samples were stained with Coomassie brilliant blue. (F) Gel filtration analysis of purified Regnase-1 in E. The molecular weight of each elution peak was estimated by using molecular weight standard markers and defined as multimer (Mw: ∞), trimer (Mw: 200 kD), or monomer (Mw: 60 kD). Mw, molecular weight.