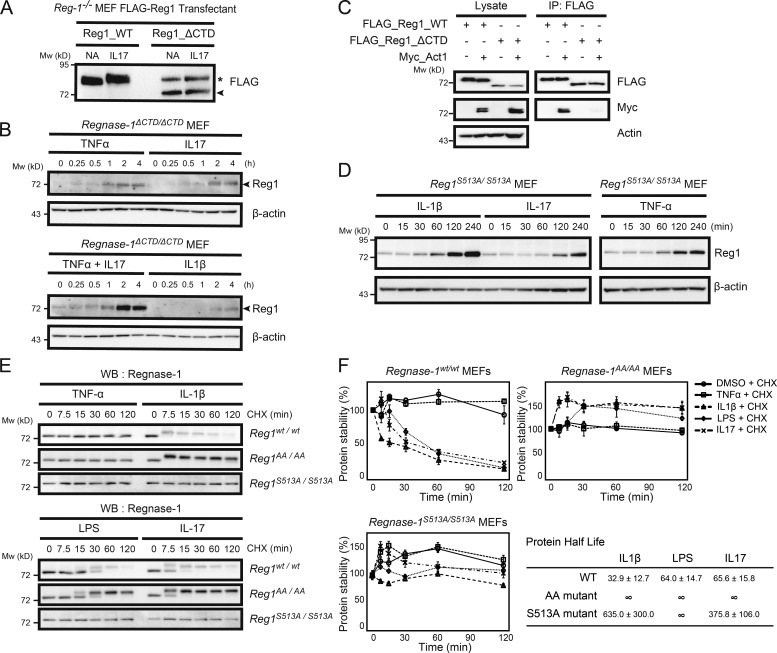
Figure 7.
Regnase-1 frameshift mutation and alanine mutation at the TBK1/IKKi phosphorylation site provide resistance to Regnase-1 degradation induced by proinflammatory cytokine stimulation. (A) Immunoblotting of lysates of Regnase-1-deficient (Reg-1–/–) MEFs stably expressing FLAG-tagged Regnase-1 (Reg1; WT and ΔCTD mutant). Cells were stimulated with or without IL-17A for 1 h and probed with anti-FLAG antibody. Regnase-1ΔCTD mutant and nonspecific protein bands are indicated by the arrowhead and asterisk, respectively. NA, no addition. (B) Immunoblotting of Regnase-1 and β-actin in Regnase-1ΔCTD/ΔCTD MEFs stimulated as indicated for 0–4 h. Arrowheads indicate Regnase-1. (C) Coimmunoprecipitation of FLAG-tagged WT Regnase-1 or the ΔCTD mutant with Act1, TBK1, and IKKi in HEK293 cells. Immunoprecipitated Regnase-1 was subjected to immunoblotting as indicated. IP, immunoprecipitation. (D) Immunoblotting analysis of Regnase-1 and β-actin in Regnase-1S51A/S513A MEFs stimulated with IL-1β, IL-17, and TNF-α for 0–4 h. (E and F) Comparison of protein half-life of Regnase-1 variants. Regnase-1wt/wt, Regnase-1AA/AA, and Regnase-1S513A/S513A MEFs were exposed to cycloheximide (CHX) and stimulated with TNF-α, IL-1β, LPS, and IL-17 for 0–120 min. (E) Immunoblotting analysis of Regnase-1 after stimulation as indicated. WB, western blotting. (F) Regnase-1 protein measured by immunoblotting and normalized to β-actin control. Protein half-lives are shown. Data were collected from three independent experiments. Mw, molecular weight.